The cell cycle, or cell-division cycle, is the series of events that take place in a cell that causes it to divide into two daughter cells. These events include the duplication of its DNA and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division.
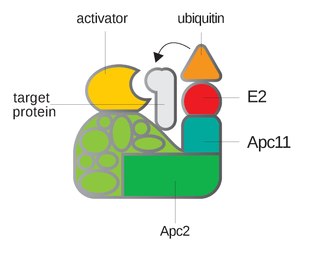
Anaphase-promoting complex is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin (Apc2) and RING (Apc11) subunit much like SCF. Other parts of the APC/C have unknown functions but are highly conserved.
Cyclin is a family of proteins that controls the progression of a cell through the cell cycle by activating cyclin-dependent kinase (CDK) enzymes or group of enzymes required for synthesis of cell cycle.

Cyclin-dependent kinases (CDKs) are the families of protein kinases first discovered for their role in regulating the cell cycle. They are also involved in regulating transcription, mRNA processing, and the differentiation of nerve cells. They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved. In fact, yeast cells can proliferate normally when their CDK gene has been replaced with the homologous human gene. CDKs are relatively small proteins, with molecular weights ranging from 34 to 40 kDa, and contain little more than the kinase domain. By definition, a CDK binds a regulatory protein called a cyclin. Without cyclin, CDK has little kinase activity; only the cyclin-CDK complex is an active kinase but its activity can be typically further modulated by phosphorylation and other binding proteins, like p27. CDKs phosphorylate their substrates on serines and threonines, so they are serine-threonine kinases. The consensus sequence for the phosphorylation site in the amino acid sequence of a CDK substrate is [S/T*]PX[K/R], where S/T* is the phosphorylated serine or threonine, P is proline, X is any amino acid, K is lysine, and R is arginine.
A cyclin-dependent kinase complex is a protein complex formed by the association of an inactive catalytic subunit of a protein kinase, cyclin-dependent kinase (CDK), with a regulatory subunit, cyclin. Once cyclin-dependent kinases bind to cyclin, the formed complex is in an activated state. Substrate specificity of the activated complex is mainly established by the associated cyclin within the complex. Activity of CDKCs is controlled by phosphorylation of target proteins, as well as binding of inhibitory proteins.

S phase (Synthesis phase) is the phase of the cell cycle in which DNA is replicated, occurring between G1 phase and G2 phase. Since accurate duplication of the genome is critical to successful cell division, the processes that occur during S-phase are tightly regulated and widely conserved.
Cdc25 is a dual-specificity phosphatase first isolated from the yeast Schizosaccharomyces pombe as a cell cycle defective mutant. As with other cell cycle proteins or genes such as Cdc2 and Cdc4, the "cdc" in its name refers to "cell division cycle". Dual-specificity phosphatases are considered a sub-class of protein tyrosine phosphatases. By removing inhibitory phosphate residues from target cyclin-dependent kinases (Cdks), Cdc25 proteins control entry into and progression through various phases of the cell cycle, including mitosis and S ("Synthesis") phase.

Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, with progression through the various phases of the cell cycle occurring only when favorable conditions are met. There are many checkpoints in the cell cycle, but the three major ones are: the G1 checkpoint, also known as the Start or restriction checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint. Progression through these checkpoints is largely determined by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell cycle to control the specific events that occur therein.

CDK-activating kinase (CAK) activates the cyclin-CDK complex by phosphorylating threonine residue 160 in the CDK activation loop. CAK itself is a member of the Cdk family and functions as a positive regulator of Cdk1, Cdk2, Cdk4, and Cdk6.
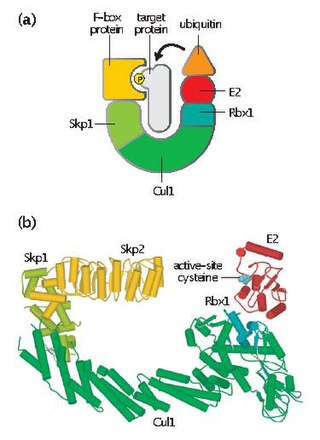
Skp, Cullin, F-box containing complex is a multi-protein E3 ubiquitin ligase complex that catalyzes the ubiquitination of proteins destined for 26S proteasomal degradation. Along with the anaphase-promoting complex, SCF has important roles in the ubiquitination of proteins involved in the cell cycle. The SCF complex also marks various other cellular proteins for destruction.

A cyclin-dependent kinase inhibitor protein(also known as CKIs, CDIs, or CDKIs) is a protein which inhibits the enzyme cyclin-dependent kinase (CDK) and Cyclin activity by stopping the cell cycle if there are unfavorable conditions, therefore, acting as tumor suppressors. Cell cycle progression is stopped by Cyclin-dependent kinase inhibitor protein at the G1 phase. CKIs are vital proteins within the control system that point out whether the process of DNA synthesis, mitosis, and cytokines control one another. If a malfunction prevents the successful completion of DNA synthesis during the G1 phase, a signal is sent to delay or stop the progression to the S phase. Cyclin-dependent kinase inhibitor proteins are essential in the regulation of the cell cycle. If cell mutations surpass the cell cycle checkpoints during cell cycle regulation, it can result in various types of cancer.

Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine protein kinase, and is a key player in cell cycle regulation. It has been highly studied in the budding yeast S. cerevisiae, and the fission yeast S. pombe, where it is encoded by genes cdc28 and cdc2, respectively. With its cyclin partners, Cdk1 forms complexes that phosphorylate a variety of target substrates ; phosphorylation of these proteins leads to cell cycle progression.

Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B beta isoform is an enzyme that in humans is encoded by the PPP2R2B gene.

Cyclin-dependent kinases regulatory subunit 1 is a protein that in humans is encoded by the CKS1B gene.

Cyclin-dependent kinases regulatory subunit 2 is a protein that in humans is encoded by the CKS2 gene.

Cyclin-G2 is a protein that in humans is encoded by the CCNG2 gene.
Sic1, a protein, is a stoichiometric inhibitor of Cdk1-Clb complexes in the budding yeast Saccharomyces cerevisiae. Because B-type cyclin-Cdk1 complexes are the drivers of S-phase initiation, Sic1 prevents premature S-phase entry. Multisite phosphorylation of Sic1 is thought to time Sic1 ubiquitination and destruction, and by extension, the timing of S-phase entry.

Wee1 is a nuclear kinase belonging to the Ser/Thr family of protein kinases in the fission yeast Schizosaccharomyces pombe. Wee1 has a molecular mass of 96 kDa and is a key regulator of cell cycle progression. It influences cell size by inhibiting the entry into mitosis, through inhibiting Cdk1. Wee1 has homologues in many other organisms, including mammals.

Cdc4 is a substrate recognition component of the SCF ubiquitin ligase complex, which acts as a mediator of ubiquitin transfer to target proteins, leading to their subsequent degradation via the ubiquitin-proteasome pathway. Cdc4 targets primarily cell cycle regulators for proteolysis. It serves the function of an adaptor that brings target molecules to the core SCF complex. Cdc4 was originally identified in the model organism Saccharomyces cerevisiae. CDC4 gene function is required at G1/S and G2/M transitions during mitosis and at various stages during meiosis.

In cell biology, eukaryotes possess a regulatory system that ensures that DNA replication occurs only once per cell cycle.