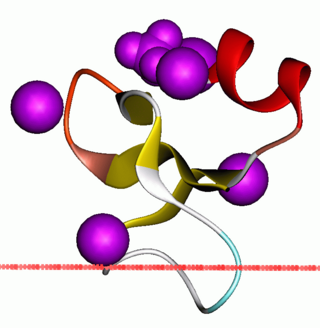
Defensin, alpha 6 (DEFA6) also known as human alpha defensin 6 (HD6) is a human protein that is encoded by the DEFA6 gene. [3] [4] DEFA6 is expressed in the Paneth cells of the ileum. [5]
Defensin, alpha 6 (DEFA6) also known as human alpha defensin 6 (HD6) is a human protein that is encoded by the DEFA6 gene. [3] [4] DEFA6 is expressed in the Paneth cells of the ileum. [5]
The alpha defensins are a family of microbicidal and cytotoxic peptides that defend the host against bacteria and viruses. HD6 has poor antibacterial potency. [6] However, HD6 affords protection against invasion by enteric bacterial pathogens by self-assembly to form fibrils and nanonets that surround and entangle bacteria. [7]
Several alpha defensin genes, including DEFA6, are clustered on chromosome 8. [3]

Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune signaling activities, or both. They are variously active against bacteria, fungi and many enveloped and nonenveloped viruses. They are typically 18-45 amino acids in length, with three or four highly conserved disulphide bonds.

Paneth cells are cells in the small intestine epithelium, alongside goblet cells, enterocytes, and enteroendocrine cells. Some can also be found in the cecum and appendix. They are located below the intestinal stem cells in the intestinal glands and the large eosinophilic refractile granules that occupy most of their cytoplasm.

Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the CAMP gene encodes the peptide precursor CAP-18, which is processed by proteinase 3-mediated extracellular cleavage into the active form LL-37. LL-37 is the only peptide in the Cathelicidin family found in the human body.

Beta-defensin 2 (BD-2) also known as skin-antimicrobial peptide 1 (SAP1) is a peptide that in humans is encoded by the DEFB4 gene.

Alpha defensins are a family of mammalian defensin peptides of the alpha subfamily. In mammals they are also known as cryptdins and are produced within the small bowel. Cryptdin is a portmanteau of crypt and defensin.
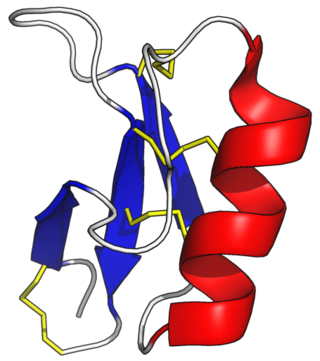
Beta defensins are a family of vertebrate defensins. The beta defensins are antimicrobial peptides implicated in the resistance of epithelial surfaces to microbial colonization.

Defensin, alpha 1 also known as human alpha defensin 1, human neutrophil peptide 1 (HNP-1) or neutrophil defensin 1 is a human protein that is encoded by the DEFA1 gene. Human alpha defensin 1 belongs to the alpha defensin family of antimicrobial peptides.

Beta-defensin 1 is a protein that in humans is encoded by the DEFB1 gene.

Beta-defensin 103 is a protein that in humans is encoded by the DEFB103A gene.
Plant defensins are a family of small, cysteine-rich defensins found in plants that serve to defend them against pathogens and parasites.
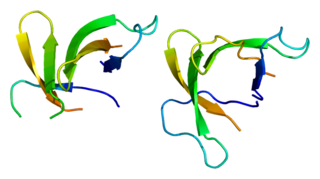
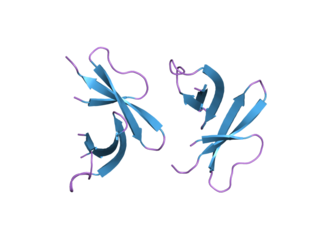
Defensin, alpha 5 (DEFA5) also known as human alpha defensin 5 (HD5) is a protein that in humans is encoded by the DEFA5 gene. DEFA5 is expressed in the Paneth cells of the ileum.

Beta-defensin 104 is a protein that in humans is encoded by the DEFB104A gene.

Beta-defensin 106 is a protein that in humans is encoded by the DEFB106A gene.
Protegrins are small peptides containing 16-18 amino acid residues. Protegrins were first discovered in porcine leukocytes and were found to have antimicrobial activity against bacteria, fungi, and some enveloped viruses. The amino acid composition of protegrins contains six positively charged arginine residues and four cysteine residues. Their secondary structure is classified as cysteine-rich β-sheet antimicrobial peptides, AMPs, that display limited sequence similarity to certain defensins and tachyplesins. In solution, the peptides fold to form an anti-parallel β-strand with the structure stabilized by two cysteine bridges formed among the four cysteine residues. Recent studies suggest that protegrins can bind to lipopolysaccharide, a property that may help them to insert into the membranes of gram-negative bacteria and permeabilize them.
Defensin, alpha 4 (DEFA4), also known as neutrophil defensin 4 or HNP4, is a human defensin peptide that is encoded by the DEFA4 gene. HNP4 is expressed in the granules of the neutrophil where it defends the host against bacteria and viruses.

Defensin, alpha 1B a human protein that is encoded by the DEFA1B gene.
Defensin, alpha 3 (DEFA3) also known as human alpha defensin 3, human neutrophil peptide 3 (HNP-3) or neutrophil defensin 3 is a human protein that is encoded by the DEFA3 gene. Human alpha defensin 3 belongs to the alpha defensin family of antimicrobial peptides.
Theta-defensins are a family of mammalian antimicrobial peptides. They are found in non-human 'Old World' primates, but not in human, gorilla, bonobo, and chimpanzee.
A nanonet is a net with fibers on the scale of nanometers. The net can be composed of carbon, metals, silicon, or peptides, such as nanonets composed of the defensin HD6. The word nanonet is also used in reference to a nanoscale communication network, which also uses key components on the scale of a hundred nanometers as officially defined in IEEE P1906.1.
Virtual colony count (VCC) is a kinetic, 96-well microbiological assay originally developed to measure the activity of defensins. It has since been applied to other antimicrobial peptides including LL-37. It utilizes a method of enumerating bacteria called quantitative growth kinetics, which compares the time taken for a bacterial batch culture to reach a threshold optical density with that of a series of calibration curves. The name VCC has also been used to describe the application of quantitative growth kinetics to enumerate bacteria in cell culture infection models. Antimicrobial susceptibility testing (AST) can be done on 96-well plates by diluting the antimicrobial agent at varying concentrations in broth inoculated with bacteria and measuring the minimum inhibitory concentration that results in no growth. However, these methods cannot be used to study some membrane-active antimicrobial peptides, which are inhibited by the broth itself. The virtual colony count procedure takes advantage of this fact by first exposing bacterial cells to the active antimicrobial agent in a low-salt buffer for two hours, then simultaneously inhibiting antimicrobial activity and inducing exponential growth by adding broth. The growth kinetics of surviving cells can then be monitored using a temperature-controlled plate reader. The time taken for each growth curve to reach a threshold change in optical density is then converted into virtual survival values, which serve as a measure of antimicrobial activity.