Related Research Articles
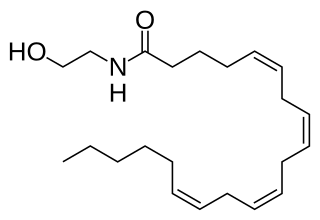
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), an N-acylethanolamine (NAE), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", plus amide.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands:
Depolarization-induced suppression of inhibition is the classical and original electrophysiological example of endocannabinoid function in the central nervous system. Prior to the demonstration that depolarization-induced suppression of inhibition was dependent on the cannabinoid CB1 receptor function, there was no way of producing an in vitro endocannabinoid mediated effect.

URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH). FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH leads to an accumulation of anandamide in the CNS and periphery where it activates cannabinoid receptors. URB597 has been found to elevate anandamide levels and have activity against neuropathic pain in a mouse model.

URB754 was originally reported by Piomelli et al. to be a potent, noncompetitive inhibitor of monoacylglycerol lipase (MGL). However, recent studies have shown that URB754 failed to inhibit recombinant MGL, and brain FAAH activity was also resistant to URB754. In a later study by Piomelli et al., the MGL-inhibitory activity attributed to URB754 is in fact due to a chemical impurity present in the commercial sample, identified as bis(methylthio)mercurane.

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system and peripheral nervous system. The endocannabinoid system remains under preliminary research, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

Monoacylglycerol lipase is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.

2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor. It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk. The chemical was first described in 1994–1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation. 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.

Oleoylethanolamide (OEA) is an endogenous peroxisome proliferator-activated receptor alpha (PPAR-α) agonist. It is a naturally occurring ethanolamide lipid that regulates feeding and body weight in vertebrates ranging from mice to pythons.

An N-acylethanolamine (NAE) is a type of fatty acid amide where one of several types of acyl groups is linked to the nitrogen atom of ethanolamine, and highly metabolic formed by intake of essential fatty acids through diet by 20:4, n-6 and 22:6, n-3 fatty acids, and when the body is physically and psychologically active,. The endocannabinoid signaling system (ECS) is the major pathway by which NAEs exerts its physiological effects in animal cells with similarities in plants, and the metabolism of NAEs is an integral part of the ECS, a very ancient signaling system, being clearly present from the divergence of the protostomian/deuterostomian, and even further back in time, to the very beginning of bacteria, the oldest organisms on Earth known to express phosphatidylethanolamine, the precursor to endocannabinoids, in their cytoplasmic membranes. Fatty acid metabolites with affinity for CB receptors are produced by cyanobacteria, which diverged from eukaryotes at least 2000 Million years ago (MYA), by brown algae which diverged about 1500 MYA, by sponges, which diverged from eumetazoans about 930 MYA, and a lineages that predate the evolution of CB receptors, as CB1 – CB2 duplication event may have occurred prior to the lophotrochozoan-deuterostome divergence 590 MYA. Fatty acid amide hydrolase (FAAH) evolved relatively recently, either after the evolution of fish 400 MYA, or after the appearance of mammals 300 MYA, but after the appearance of vertebrates. Linking FAAH, vanilloid receptors (VR1) and anandamide implies a coupling among the remaining ‘‘older’’ parts of the endocannabinoid system, monoglyceride lipase (MGL), CB receptors, that evolved prior to the metazoan–bilaterian divergence, but were secondarily lost in the Ecdysozoa, and 2-Arachidonoylglycerol (2-AG).
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.

Abnormal cannabidiol (Abn-CBD) is a synthetic regioisomer of cannabidiol, which unlike most other cannabinoids produces vasodilator effects, lowers blood pressure, and induces cell migration, cell proliferation and mitogen-activated protein kinase activation in microglia, but without producing any psychoactive or sedative effects.

URB602 is a compound that has been found to inhibit hydrolysis of monoacyl glycerol compounds, such as 2-arachidonoylglycerol (2-AG) and 2-oleoylglycerol (2-OG). It was first described in 2003. A study performed in 2005 found that the compound had specificity for metabolizing 2-AG over anandamide in rat brain presumably by inhibiting the enzyme monoacylglycerol lipase (MAGL), which is the primary metabolic enzyme of 2-AG. However, subsequent studies have shown that URB602 lacks specificity for MAGL inhibition in vitro.

N-Arachidonylglycine (NAGly) is a carboxylic metabolite of the endocannabinoid anandamide (AEA). Since it was first synthesized in 1996, NAGly has been a primary focus of the relatively contemporary field of lipidomics due to its wide range of signaling targets in the brain, the immune system and throughout various other bodily systems. In combination with 2‐arachidonoyl glycerol (2‐AG), NAGly has enabled the identification of a family of lipids often referred to as endocannabinoids. Recently, NAGly has been found to bind to G-protein coupled receptor 18 (GPR18), the putative abnormal cannabidiol receptor. NaGly is an endogenous inhibitor of fatty acid amide hydrolase (FAAH) and thereby increases the ethanolamide endocannabinoids AEA, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels. NaGly is found throughout the body and research on its explicit functions is ongoing.
The endocannabinoid transporters (eCBTs) are transport proteins for the endocannabinoids. Most neurotransmitters are water-soluble and require transmembrane proteins to transport them across the cell membrane. The endocannabinoids on the other hand, are non-charged lipids that readily cross lipid membranes. However, since the endocannabinoids are water immiscible, protein transporters have been described that act as carriers to solubilize and transport the endocannabinoids through the aqueous cytoplasm. These include the heat shock proteins (Hsp70s) and fatty acid-binding proteins for anandamide (FABPs). FABPs such as FABP1, FABP3, FABP5, and FABP7 have been shown to bind endocannabinoids. FABP inhibitors attenuate the breakdown of anandamide by the enzyme fatty acid amide hydrolase (FAAH) in cell culture. One of these inhibitors (SB-FI-26), isolated from a virtual library of a million compounds, belongs to a class of compounds that act as an anti-nociceptive agent with mild anti-inflammatory activity in mice. These truxillic acids and their derivatives have been known to have anti-inflammatory and anti-nociceptive effects in mice and are active components of a Chinese herbal medicine used to treat rheumatism and pain in human. The blockade of anandamide transport may, at least in part, be the mechanism through which these compounds exert their anti-nociceptive effects.
N-acylethanolamine acid amide hydrolase (NAAA) EC 3.5.1.- is a member of the choloylglycine hydrolase family, a subset of the N-terminal nucleophile hydrolase superfamily. NAAA has a molecular weight of 31 kDa. The activation and inhibition of its catalytic site is of medical interest as a potential treatment for obesity and chronic pain. While it was discovered within the last decade, its structural similarity to the more familiar acid ceramidase (AC) and functional similarity to fatty acid amide hydrolase (FAAH) allow it to be studied extensively.
An endocannabinoid enhancer (eCBE) is a type of cannabinoidergic drug that enhances the activity of the endocannabinoid system by increasing extracellular concentrations of endocannabinoids. Examples of different types of eCBEs include fatty acid amide hydrolase (FAAH) inhibitors, monoacylglycerol lipase (MAGL) inhibitors, and endocannabinoid transporter (eCBT) inhibitors. An example of an actual eCBE is AM404, the active metabolite of the analgesic paracetamol and a dual FAAH inhibitor and eCBRI.
References
- ↑ Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D (1994). “Formation and inactivation of endogenous cannabinoid anandamide in central neurons”. Nature 372 (6507): 686-91. PMID 7990962.
- ↑ Stella N, Schweitzer P, Piomelli, D (1997). “A second endogenous cannabinoid that modulates long-term potentiation”. Nature 388 (6644): 773-8. PMID 9285589.
- ↑ Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D (1999). “Dopamine activation of endogenous cannabinoid signaling in dorsal striatum”. Nature Neuroscience 2 (4): 358-63. PMID 10204543.
- ↑ Calignano A, Katona I, Desarnaud F, Giuffrida A, La Rana G, Mackie K, Freund TF, Piomelli D (2000). “Bidirectional control of airway responsiveness by endogenous cannabinoids”. Nature 408 (6808): 96-101. PMID 11081515.
- ↑ Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2005). “An endocannabinoid mechanism for stress-induced analgesia”. Nature 435 (7045): 1108-12. PMID 15973410.
- ↑ Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D (2010). “Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism”. Nature Neuroscience 13 (10): 1265-70. PMID 20852626.
- ↑ DiPatrizio NV, Astarita G, Schwartz GJ, Li X, Piomelli D (2011) “Endocannabinoid signal in the gut controls dietary fat intake”. Proceedings of the National Academy of Sciences USA 108 (31): 12904-8. PMID 21730161.
- ↑ Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomellli D, Manzoni OJ (2013) “Uncoupling of the endocannabinoid signaling complex in a mouse model of fragile X syndrome”. Nature Communications 3 (1080). PMID 23011134.
- ↑ Calignano A, La Rana G, Makriyannis A, Lin SY, Beltramo M, Piomelli D (1998) “Control of pain initiation by endogenous cannabinoids”. Nature 394 (6690): 277-81. PMID 9685157.
- ↑ Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, Lo Verme J, Gaetani S, Kathuria S, Gall C, Piomelli D (2001) “An anorexic lipid mediator regulated by feeding”. Nature 414 (6860): 209-12. PMID 11700558.
- ↑ Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez de Fonseca F, Rosengarth A, Lueke H, Di Giacomo B, Tarzia G, Piomelli D (2003) “Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha”. Nature 425 (6953): 90-3. PMID 12955147.
- ↑ Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, Cuomo V, Piomelli D (2008) “The lipid messenger OEA links dietary fat intake to satiety” Cell Metabolism 8 (4): 281-8. PMID 18840358.
- ↑ Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez de Fonseca F, Rosengarth A, Lueke H, Di Giacomo B, Tarzia G, Piomelli D (2003) “Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha”. Nature 425 (6953): 90-3. PMID 12955147.
- ↑ Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D (2005) “The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide”. Molecular Pharmacology 67 (1): 15-9. PMID 15465922.
- ↑ Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D (2003) “Modulation of anxiety through blockade of anandamide hydrolysis”. Nature Medicine 9 (1): 76-81. PMID 12461523.
- ↑ Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D (2006) “Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis”. Proceedings of the National Academy of Sciences USA 103 (7): 24665. PMID 16352709.
- ↑ Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, Rivara S, Mor M, Russo R, Maccarrone M, Antonietti F, Duranti A, Tontini A, Cuzzocrea S, Tarzia G, Piomelli D (2009) “Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation”. Proceedings of the National Academy of Sciences USA 106 (49): 20966-71. PMID 19926854.
- ↑ Realini N, Solorzano C, Pagliuca C, Pizzirani D, Armirotti A, Luciani R, Costi MP, Bandiera T, Piomelli D (2013) “Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity”. Science Reports 3:1035. PMID 23301156.