Related Research Articles

Aortic stenosis is the narrowing of the exit of the left ventricle of the heart, such that problems result. It may occur at the aortic valve as well as above and below this level. It typically gets worse over time. Symptoms often come on gradually with a decreased ability to exercise often occurring first. If heart failure, loss of consciousness, or heart related chest pain occur due to AS the outcomes are worse. Loss of consciousness typically occurs with standing or exercising. Signs of heart failure include shortness of breath especially when lying down, at night, or with exercise, and swelling of the legs. Thickening of the valve without narrowing is known as aortic sclerosis.
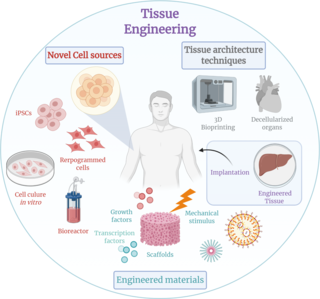
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own.

An artificial heart valve is a one-way valve implanted into a person's heart to replace a heart valve that is not functioning properly. Artificial heart valves can be separated into three broad classes: mechanical heart valves, bioprosthetic tissue valves and engineered tissue valves.
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science.

A foreign body reaction (FBR) is a typical tissue response to a foreign body within biological tissue. It usually includes the formation of a foreign body granuloma. Tissue-encapsulation of an implant is an example, as is inflammation around a splinter. Foreign body granuloma formation consists of protein adsorption, macrophages, multinucleated foreign body giant cells, fibroblasts, and angiogenesis. It has also been proposed that the mechanical property of the interface between an implant and its surrounding tissues is critical for the host response.
Neural tissue engineering is a specific sub-field of tissue engineering. Neural tissue engineering is primarily a search for strategies to eliminate inflammation and fibrosis upon implantation of foreign substances. Often foreign substances in the form of grafts and scaffolds are implanted to promote nerve regeneration and to repair damage caused to nerves of both the central nervous system (CNS) and peripheral nervous system (PNS) by an injury.
A nerve guidance conduit is an artificial means of guiding axonal regrowth to facilitate nerve regeneration and is one of several clinical treatments for nerve injuries. When direct suturing of the two stumps of a severed nerve cannot be accomplished without tension, the standard clinical treatment for peripheral nerve injuries is autologous nerve grafting. Due to the limited availability of donor tissue and functional recovery in autologous nerve grafting, neural tissue engineering research has focused on the development of bioartificial nerve guidance conduits as an alternative treatment, especially for large defects. Similar techniques are also being explored for nerve repair in the spinal cord but nerve regeneration in the central nervous system poses a greater challenge because its axons do not regenerate appreciably in their native environment.
Nano-scaffolding or nanoscaffolding is a medical process used to regrow tissue and bone, including limbs and organs. The nano-scaffold is a three-dimensional structure composed of polymer fibers very small that are scaled from a Nanometer scale. Developed by the American military, the medical technology uses a microscopic apparatus made of fine polymer fibers called a scaffold. Damaged cells grip to the scaffold and begin to rebuild missing bone and tissue through tiny holes in the scaffold. As tissue grows, the scaffold is absorbed into the body and disappears completely.
A fibrin scaffold is a network of protein that holds together and supports a variety of living tissues. It is produced naturally by the body after injury, but also can be engineered as a tissue substitute to speed healing. The scaffold consists of naturally occurring biomaterials composed of a cross-linked fibrin network and has a broad use in biomedical applications.
Acellular dermis is a type of biomaterial derived from processing human or animal tissues to remove cells and retain portions of the extracellular matrix (ECM). These materials are typically cell-free, distinguishing them from classical allografts and xenografts, can be integrated or incorporated into the body, and have been FDA approved for human use for more than 10 years in a wide range of clinical indications.

Biomaterials are materials that are used in contact with biological systems. Biocompatibility and applicability of surface modification with current uses of metallic, polymeric and ceramic biomaterials allow alteration of properties to enhance performance in a biological environment while retaining bulk properties of the desired device.

Decellularization is the process used in biomedical engineering to isolate the extracellular matrix (ECM) of a tissue from its inhabiting cells, leaving an ECM scaffold of the original tissue, which can be used in artificial organ and tissue regeneration. Organ and tissue transplantation treat a variety of medical problems, ranging from end organ failure to cosmetic surgery. One of the greatest limitations to organ transplantation derives from organ rejection caused by antibodies of the transplant recipient reacting to donor antigens on cell surfaces within the donor organ. Because of unfavorable immune responses, transplant patients suffer a lifetime taking immunosuppressing medication. Stephen F. Badylak pioneered the process of decellularization at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. This process creates a natural biomaterial to act as a scaffold for cell growth, differentiation and tissue development. By recellularizing an ECM scaffold with a patient’s own cells, the adverse immune response is eliminated. Nowadays, commercially available ECM scaffolds are available for a wide variety of tissue engineering. Using peracetic acid to decellularize ECM scaffolds have been found to be false and only disinfects the tissue.
A bioartificial heart is an engineered heart that contains the extracellular structure of a decellularized heart and cellular components from a different source. Such hearts are of particular interest for therapy as well as research into heart disease. The first bioartificial hearts were created in 2008 using cadaveric rat hearts. In 2014, human-sized bioartificial pig hearts were constructed. Bioartificial hearts have not been developed yet for clinical use, although the recellularization of porcine hearts with human cells opens the door to xenotransplantation.

Decellularized homografts are donated human heart valves which have been modified via tissue engineering. Several techniques exist for decellularization with the majority based on detergent or enzymatic protocols which aim to eliminate all donor cells while preserving the mechanical properties of the remaining matrix.
In vitro models for calcification may refer to systems that have been developed in order to reproduce, in the best possible way, the calcification process that tissues or biomaterials undergo inside the body. The aim of these systems is to mimic the high levels of calcium and phosphate present in the blood and measure the extent of the crystal's deposition. Different variations can include other parameters to increase the veracity of these models, such as flow, pressure, compliance and resistance. All the systems have different limitations that have to be acknowledged regarding the operating conditions and the degree of representation. The rational of using such is to partially replace in vivo animal testing, whilst rendering much more controllable and independent parameters compared to an animal model.
Bio-inks are materials used to produce engineered/artificial live tissue using 3D printing. These inks are mostly composed of the cells that are being used, but are often used in tandem with additional materials that envelope the cells. The combination of cells and usually biopolymer gels are defined as a bio-ink. They must meet certain characteristics, including such as rheological, mechanical, biofunctional and biocompatibility properties, among others. Using bio-inks provides a high reproducibility and precise control over the fabricated constructs in an automated manner. These inks are considered as one of the most advanced tools for tissue engineering and regenerative medicine (TERM).

Antonios Georgios Mikos is a Greek-American biomedical engineer who is the Louis Calder Professor of Bioengineering and Chemical and Biomolecular Engineering at Rice University. He specialises in biomaterials, drug delivery, and tissue engineering.
Tissue engineered heart valves (TEHV) offer a new and advancing proposed treatment of creating a living heart valve for people who are in need of either a full or partial heart valve replacement. Currently, there are over a quarter of a million prosthetic heart valves implanted annually, and the number of patients requiring replacement surgeries is only suspected to rise and even triple over the next fifty years. While current treatments offered such as mechanical valves or biological valves are not deleterious to one's health, they both have their own limitations in that mechanical valves necessitate the lifelong use of anticoagulants while biological valves are susceptible to structural degradation and reoperation. Thus, in situ (in its original position or place) tissue engineering of heart valves serves as a novel approach that explores the use creating a living heart valve composed of the host's own cells that is capable of growing, adapting, and interacting within the human body's biological system.
Katja Schenke-Layland is the Professor of Medical Technologies and Regenerative Medicine, Institute of Biomedical Engineering, Department for Medical Technologies and Regenerative Medicine at the University of Tübingen. She is the Director of the NMI Natural and Medical Sciences Institute at the University Tübingen in Reutlingen, Study Dean of Medical Technologies at the University of Tübingen, and Founding Director of the Institute of Biomedical Engineering at the Medical Faculty of the University Tübingen. She is also the Founding Director of the 3R Center for In Vitro Models and Alternatives to Animal Testing Tübingen.
Kristyn Simcha Masters is an American bioengineer who is professor and Chair of the Department of Bioengineering at the University of Colorado Denver. She works as Director of the Anschutz Medical Campus Center. Her research looks to create tissue-engineered models of disease, with a focus on cancer and cardiac disease.
References
- ↑ Cebotari S, Tudorache I, Jaekel T, Hilfiker A, Dorfman S, Ternes W, et al. (March 2010). "Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells". Artificial Organs. 34 (3): 206–10. doi:10.1111/j.1525-1594.2009.00796.x. PMID 20447045.
- ↑ Kasimir MT, Rieder E, Seebacher G, Silberhumer G, Wolner E, Weigel G, et al. (May 2003). "Comparison of different decellularization procedures of porcine heart valves". The International Journal of Artificial Organs. 26 (5): 421–7. doi:10.1177/039139880302600508. PMID 12828309. S2CID 28085063.
- 1 2 3 4 Schenke-Layland K, Vasilevski O, Opitz F, König K, Riemann I, Halbhuber KJ, et al. (September 2003). "Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves". Journal of Structural Biology. 143 (3): 201–8. doi:10.1016/j.jsb.2003.08.002. PMID 14572475.
- 1 2 3 4 5 Bloch O, Golde P, Dohmen PM, Posner S, Konertz W, Erdbrügger W (October 2011). "Immune response in patients receiving a bioprosthetic heart valve: lack of response with decellularized valves". Tissue Engineering. Part A. 17 (19–20): 2399–405. doi:10.1089/ten.TEA.2011.0046. PMID 21557643. S2CID 36527124.
- 1 2 Guyton AC, Hall JE (2006). Medical Physiology (11th ed.). Pennsylvania, PHIL: Elsevier.
- 1 2 3 4 Liao J, Joyce EM, Sacks MS (March 2008). "Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet". Biomaterials. 29 (8): 1065–74. doi:10.1016/j.biomaterials.2007.11.007. PMC 2253688 . PMID 18096223.
- 1 2 3 4 5 Patnaik SS, Wang B, Weed B, Wertheim JA, Liao J (2013-02-21). "Chapter 3: Decellularized Scaffolds: Concepts, Methodologies, and Applications in Cardiac Tissue Engineering and Whole-Organ Regeneration". Tissue Regeneration. Frontiers in Nanobiomedical Research. Vol. 2. World Scientific. pp. 77–124. doi:10.1142/9789814494847_0003. ISBN 9789814494830. S2CID 28114835.
- ↑ Gallo M, Naso F, Poser H, Rossi A, Franci P, Bianco R, et al. (June 2012). "Physiological performance of a detergent decellularized heart valve implanted for 15 months in Vietnamese pigs: surgical procedure, follow-up, and explant inspection". Artificial Organs. 36 (6): E138–50. doi:10.1111/j.1525-1594.2012.01447.x. PMID 22512408.
- ↑ Rieder E, Seebacher G, Kasimir MT, Eichmair E, Winter B, Dekan B, et al. (May 2005). "Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells". Circulation. 111 (21): 2792–7. doi:10.1161/CIRCULATIONAHA.104.473629. PMID 15911701.
- ↑ Chow JP, Simionescu DT, Warner H, Wang B, Patnaik SS, Liao J, Simionescu A (January 2013). "Mitigation of diabetes-related complications in implanted collagen and elastin scaffolds using matrix-binding polyphenol". Biomaterials. 34 (3): 685–95. doi:10.1016/j.biomaterials.2012.09.081. PMC 3496025 . PMID 23103157.
- 1 2 Sierad LN, Shaw EL, Bina A, Brazile B, Rierson N, Patnaik SS, et al. (December 2015). "Functional Heart Valve Scaffolds Obtained by Complete Decellularization of Porcine Aortic Roots in a Novel Differential Pressure Gradient Perfusion System". Tissue Engineering. Part C, Methods. 21 (12): 1284–96. doi:10.1089/ten.TEC.2015.0170. PMC 4663650 . PMID 26467108.
- ↑ Bastian F, Stelzmüller ME, Kratochwill K, Kasimir MT, Simon P, Weigel G (April 2008). "IgG deposition and activation of the classical complement pathway involvement in the activation of human granulocytes by decellularized porcine heart valve tissue". Biomaterials. 29 (12): 1824–32. doi:10.1016/j.biomaterials.2008.01.005. PMID 18258297.