
An axon or nerve fiber is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.
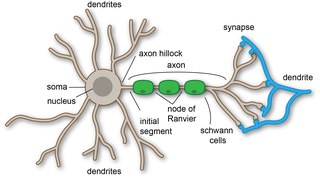
A dendrite or dendron is a branched protoplasmic extension of a nerve cell that propagates the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.

Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain and spinal cord, and the peripheral nervous system (PNS) comprising the branching peripheral nerves. It is composed of neurons, also known as nerve cells, which receive and transmit impulses, and neuroglia, also known as glial cells or glia, which assist the propagation of the nerve impulse as well as provide nutrients to the neurons.

A pseudounipolar neuron is a type of neuron which has one extension from its cell body. This type of neuron contains an axon that has split into two branches. A single process arises from the cell body and then divides into an axon and a dendrite. They develop embryologically as bipolar in shape, and are thus termed pseudounipolar instead of unipolar.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.
A basal dendrite is a dendrite that emerges from the base of a pyramidal cell that receives information from nearby neurons and passes it to the soma, or cell body. Due to their direct attachment to the cell body itself, basal dendrites are able to deliver strong depolarizing currents and therefore have a strong effect on action potential output in neurons. The physical characteristics of basal dendrites vary based on their location and species that they are found in. For example, the basal dendrites of humans are overall found to be the most intricate and spine-dense, as compared to other species such as Macaques. It is also observed that basal dendrites of the prefrontal cortex are larger and more complex in comparison to the smaller and simpler dendrites that can be seen within the visual cortex. Basal dendrites are capable of vast amounts of analog computing, which is responsible for many of the different nonlinear responses of modulating information in the neocortex. Basal dendrites additionally exist in dentate granule cells for a limited time before removal via regulatory factors. This removal usually occurs before the cell reaches adulthood, and is thought to be regulated through both intracellular and extracellular signals. Basal dendrites are part of the more overarching dendritic tree present on pyramidal neurons. They, along with apical dendrites, make up the part of the neuron that receives most of the electrical signaling. Basal dendrites have been found to be involved mostly in neocortical information processing.

The dentate nucleus is a cluster of neurons, or nerve cells, in the central nervous system that has a dentate – tooth-like or serrated – edge. It is located within the deep white matter of each cerebellar hemisphere, and it is the largest single structure linking the cerebellum to the rest of the brain. It is the largest and most lateral, or farthest from the midline, of the four pairs of deep cerebellar nuclei, the others being the globose and emboliform nuclei, which together are referred to as the interposed nucleus, and the fastigial nucleus. The dentate nucleus is responsible for the planning, initiation and control of voluntary movements. The dorsal region of the dentate nucleus contains output channels involved in motor function, which is the movement of skeletal muscle, while the ventral region contains output channels involved in nonmotor function, such as conscious thought and visuospatial function.
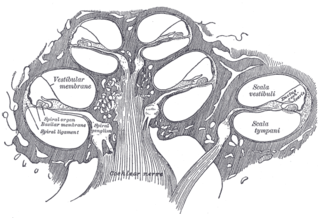
The cochlear nerve is one of two parts of the vestibulocochlear nerve, a cranial nerve present in amniotes, the other part being the vestibular nerve. The cochlear nerve carries auditory sensory information from the cochlea of the inner ear directly to the brain. The other portion of the vestibulocochlear nerve is the vestibular nerve, which carries spatial orientation information to the brain from the semicircular canals, also known as semicircular ducts.

Mitral cells are neurons that are part of the olfactory system. They are located in the olfactory bulb in the mammalian central nervous system. They receive information from the axons of olfactory receptor neurons, forming synapses in neuropils called glomeruli. Axons of the mitral cells transfer information to a number of areas in the brain, including the piriform cortex, entorhinal cortex, and amygdala. Mitral cells receive excitatory input from olfactory sensory neurons and external tufted cells on their primary dendrites, whereas inhibitory input arises either from granule cells onto their lateral dendrites and soma or from periglomerular cells onto their dendritic tuft. Mitral cells together with tufted cells form an obligatory relay for all olfactory information entering from the olfactory nerve. Mitral cell output is not a passive reflection of their input from the olfactory nerve. In mice, each mitral cell sends a single primary dendrite into a glomerulus receiving input from a population of olfactory sensory neurons expressing identical olfactory receptor proteins, yet the odor responsiveness of the 20-40 mitral cells connected to a single glomerulus is not identical to the tuning curve of the input cells, and also differs between sister mitral cells. Odorant response properties of individual neurons in an olfactory glomerular module. The exact type of processing that mitral cells perform with their inputs is still a matter of controversy. One prominent hypothesis is that mitral cells encode the strength of an olfactory input into their firing phases relative to the sniff cycle. A second hypothesis is that the olfactory bulb network acts as a dynamical system that decorrelates to differentiate between representations of highly similar odorants over time. Support for the second hypothesis comes primarily from research in zebrafish.
Dendrite derives from the Greek word "dendron" meaning, and may refer to:

The cochlear nuclear (CN) complex comprises two cranial nerve nuclei in the human brainstem, the ventral cochlear nucleus (VCN) and the dorsal cochlear nucleus (DCN). The ventral cochlear nucleus is unlayered whereas the dorsal cochlear nucleus is layered. Auditory nerve fibers, fibers that travel through the auditory nerve carry information from the inner ear, the cochlea, on the same side of the head, to the nerve root in the ventral cochlear nucleus. At the nerve root the fibers branch to innervate the ventral cochlear nucleus and the deep layer of the dorsal cochlear nucleus. All acoustic information thus enters the brain through the cochlear nuclei, where the processing of acoustic information begins. The outputs from the cochlear nuclei are received in higher regions of the auditory brainstem.

In the anatomy of the eye, the inner nuclear layer or layer of inner granules, of the retina, is made up of a number of closely packed cells, of which there are three varieties: bipolar cells, horizontal cells, and amacrine cells.

In the ventral cochlear nucleus (VCN), auditory nerve fibers enter the brain via the nerve root in the VCN. The ventral cochlear nucleus is divided into the anterior ventral (anteroventral) cochlear nucleus (AVCN) and the posterior ventral (posteroventral) cochlear nucleus (PVCN). In the VCN, auditory nerve fibers bifurcate, the ascending branch innervates the AVCN and the descending branch innervates the PVCN and then continue to the dorsal cochlear nucleus. The orderly innervation by auditory nerve fibers gives the AVCN a tonotopic organization along the dorsoventral axis. Fibers that carry information from the apex of the cochlea that are tuned to low frequencies contact neurons in the ventral part of the AVCN; those that carry information from the base of the cochlea that are tuned to high frequencies contact neurons in the dorsal part of the AVCN. Several populations of neurons populate the AVCN. Bushy cells receive input from auditory nerve fibers through particularly large endings called end bulbs of Held. They contact stellate cells through more conventional boutons.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In primate brains, including humans, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.
Kenyon cells are the intrinsic neurons of the mushroom body, a neuropil found in the brains of most arthropods and some annelids. They were first described by F. C. Kenyon in 1896. The number of Kenyon cells in an organism varies greatly between species. For example, in the fruit fly, Drosophila melanogaster, there are about 2,500 Kenyon cells per mushroom body, while in cockroaches there are about 230,000.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.
Sholl analysis is a method of quantitative analysis commonly used in neuronal studies to characterize the morphological characteristics of an imaged neuron, first used to describe the differences in the visual and motor cortices of cats in the early 1950s. Sholl was interested in comparing the morphology of different types of neurons, such as the star-shaped stellate cells and the cone-shaped pyramidal cells, and of different locations in the dendritic field of the same type of neurons, such as basal and apical processes of the pyramidal neuron. He looked at dendritic length and diameter and also the number of cells per volume.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.

Neuronal self-avoidance, or isoneural avoidance, is an important property of neurons which consists in the tendency of branches arising from a single soma to turn away from one another. The arrangements of branches within neuronal arbors are established during development and result in minimal crossing or overlap as they spread over a territory, resulting in the typical fasciculated morphology of neurons.