Related Research Articles
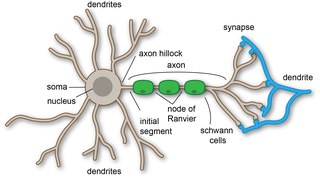
A dendrite or dendron is a branched protoplasmic extension of a nerve cell that propagates the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.

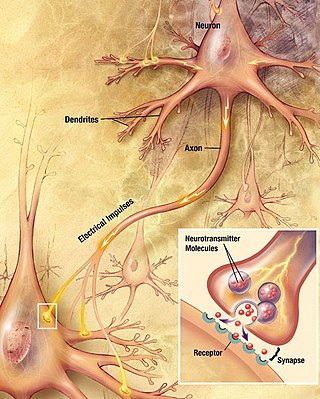
A neuron, neurone, or nerve cell is an excitable cell that fires electric signals called action potentials across a neural network in the nervous system. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.

A dendritic spine is a small membranous protrusion from a neuron's dendrite that typically receives input from a single axon at the synapse. Dendritic spines serve as a storage site for synaptic strength and help transmit electrical signals to the neuron's cell body. Most spines have a bulbous head, and a thin neck that connects the head of the spine to the shaft of the dendrite. The dendrites of a single neuron can contain hundreds to thousands of spines. In addition to spines providing an anatomical substrate for memory storage and synaptic transmission, they may also serve to increase the number of possible contacts between neurons. It has also been suggested that changes in the activity of neurons have a positive effect on spine morphology.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell-to-cell signalling. EPSPs and IPSPs compete with each other at numerous synapses of a neuron. This determines whether an action potential occurring at the presynaptic terminal produces an action potential at the postsynaptic membrane. Some common neurotransmitters involved in IPSPs are GABA and glycine.

Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal cells are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. One of the main structural features of the pyramidal neuron is the conic shaped soma, or cell body, after which the neuron is named. Other key structural features of the pyramidal cell are a single axon, a large apical dendrite, multiple basal dendrites, and the presence of dendritic spines.

The glomerulus is a spherical structure located in the olfactory bulb of the brain where synapses form between the terminals of the olfactory nerve and the dendrites of mitral, periglomerular and tufted cells. Each glomerulus is surrounded by a heterogeneous population of juxtaglomerular neurons and glial cells.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.

In neuroscience, Golgi cells are the most abundant inhibitory interneurons found within the granular layer of the cerebellum. Golgi cells can be found in the granular layer at various layers. The Golgi cell is essential for controlling the activity of the granular layer. They were first identified as inhibitory in 1964. It was also the first example of an inhibitory feedback network in which the inhibitory interneuron was identified anatomically. Golgi cells produce a wide lateral inhibition that reaches beyond the afferent synaptic field and inhibit granule cells via feedforward and feedback inhibitory loops. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

Mitral cells are neurons that are part of the olfactory system. They are located in the olfactory bulb in the mammalian central nervous system. They receive information from the axons of olfactory receptor neurons, forming synapses in neuropils called glomeruli. Axons of the mitral cells transfer information to a number of areas in the brain, including the piriform cortex, entorhinal cortex, and amygdala. Mitral cells receive excitatory input from olfactory sensory neurons and external tufted cells on their primary dendrites, whereas inhibitory input arises either from granule cells onto their lateral dendrites and soma or from periglomerular cells onto their dendritic tuft. Mitral cells together with tufted cells form an obligatory relay for all olfactory information entering from the olfactory nerve. Mitral cell output is not a passive reflection of their input from the olfactory nerve. In mice, each mitral cell sends a single primary dendrite into a glomerulus receiving input from a population of olfactory sensory neurons expressing identical olfactory receptor proteins, yet the odor responsiveness of the 20-40 mitral cells connected to a single glomerulus is not identical to the tuning curve of the input cells, and also differs between sister mitral cells. Odorant response properties of individual neurons in an olfactory glomerular module. The exact type of processing that mitral cells perform with their inputs is still a matter of controversy. One prominent hypothesis is that mitral cells encode the strength of an olfactory input into their firing phases relative to the sniff cycle. A second hypothesis is that the olfactory bulb network acts as a dynamical system that decorrelates to differentiate between representations of highly similar odorants over time. Support for the second hypothesis comes primarily from research in zebrafish.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance.

A bipolar neuron, or bipolar cell, is a type of neuron characterized by having both an axon and a dendrite extending from the soma in opposite directions. These neurons are predominantly found in the retina and olfactory system. The embryological period encompassing weeks seven through eight marks the commencement of bipolar neuron development.
Neural backpropagation is the phenomenon in which, after the action potential of a neuron creates a voltage spike down the axon, another impulse is generated from the soma and propagates towards the apical portions of the dendritic arbor or dendrites. In addition to active backpropagation of the action potential, there is also passive electrotonic spread. While there is ample evidence to prove the existence of backpropagating action potentials, the function of such action potentials and the extent to which they invade the most distal dendrites remain highly controversial.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.
Gordon Murray Shepherd was an American neuroscientist who carried out basic experimental and computational research on how neurons are organized into microcircuits to carry out the functional operations of the nervous system. Using the olfactory system as a model that spans multiple levels of space, time and disciplines, his studies ranged from molecular to behavioral, recognized by an annual lecture at Yale University on "integrative neuroscience". At the time of his death, he was professor of neuroscience emeritus at the Yale School of Medicine. He graduated from Iowa State University with a BA, Harvard Medical School with an MD, and the University of Oxford with a DPhill.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron's axon.
References
- ↑ Shepherd, G.M. (1996). "The dendritic spine: a multifunctional integrative unit". J. Neurophysiol. 75 (6): 2197–2210. doi:10.1152/jn.1996.75.6.2197. PMID 8793734.
- ↑ Masurkar, Arjun; Chen, Wei (Jan 25, 2012). "The influence of single bursts versus single spikes at excitatory dendrodendritic synapses". European Journal of Neuroscience. 35 (3): 389–401. doi:10.1111/j.1460-9568.2011.07978.x. PMC 4472665 . PMID 22277089.
- ↑ Rall, W; Shepherd, G.M.; Reese, T.S.; Brightman M.W. (January 1966). "Dendrodendritic synaptic pathway for inhibition in the olfactory bulb". Experimental Neurology. 14 (1): 44–56. doi:10.1016/0014-4886(66)90023-9. PMID 5900523.
- ↑ Shepard, G.M. (1996). "The dendritic spine: a multifunctional integrative unit". J. Neurophysiol. 75 (6): 2197–2210. doi:10.1152/jn.1996.75.6.2197. PMID 8793734.
- 1 2 Shepherd, G.M. (July 2009). "Dendrodendritic synapses: past, present and future". Annals of the New York Academy of Sciences. 1170: 215–223. doi:10.1111/j.1749-6632.2009.03937.x. PMC 3819211 . PMID 19686140.
- ↑ Hidaka, Sid; Akahori, Y.; Yoshikazu, K. (Nov 17, 2004). "Dendrodendritic Electrical Synapses between Mammalian Retinal Ganglion Cells". The Journal of Neuroscience. 24 (46): 10553–10567. doi: 10.1523/JNEUROSCI.3319-04.2004 . PMC 6730298 . PMID 15548670.
- ↑ Eggers, Arika; McCall, Maureen; Lukasiewicz, Peter (Jul 15, 2007). "Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina". The Journal of Physiology. 582 (2): 569–582. doi:10.1113/jphysiol.2007.131763. PMC 2075342 . PMID 17463042.
- 1 2 3 4 5 Hamori, J (2009). "Morphological plasticity of postsynaptic neurons in reactive synaptogenesis". J Exp Biol. 153: 251–260. doi:10.1242/jeb.153.1.251. PMID 2280223.