
An axon, or nerve fiber, is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction has caused many inherited and acquired neurological disorders which can affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.
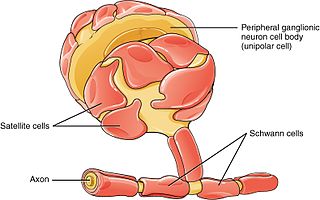
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.

Wallerian degeneration is an active process of degeneration that results when a nerve fiber is cut or crushed and the part of the axon distal to the injury degenerates. A related process of dying back or retrograde degeneration known as 'Wallerian-like degeneration' occurs in many neurodegenerative diseases, especially those where axonal transport is impaired such as ALS and Alzheimer's disease. Primary culture studies suggest that a failure to deliver sufficient quantities of the essential axonal protein NMNAT2 is a key initiating event.

Astrogliosis is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons from central nervous system (CNS) trauma, infection, ischemia, stroke, autoimmune responses or neurodegenerative disease. In healthy neural tissue, astrocytes play critical roles in energy provision, regulation of blood flow, homeostasis of extracellular fluid, homeostasis of ions and transmitters, regulation of synapse function and synaptic remodeling. Astrogliosis changes the molecular expression and morphology of astrocytes, in response to infection for example, in severe cases causing glial scar formation that may inhibit axon regeneration.

Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. It is also found in neurons of the central nervous system that have projections toward peripheral structures, such as spinal motor neurons. Its size, structure, and sequence/location of protein motifs is similar to other type III intermediate filament proteins such as desmin, vimentin and glial fibrillary acidic protein. Like these proteins, peripherin can self-assemble to form homopolymeric filamentous networks, but it can also heteropolymerize with neurofilaments in several neuronal types. This protein in humans is encoded by the PRPH gene. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury, but its exact function is unknown. It is also associated with some of the major neuropathologies that characterize amyotropic lateral sclerosis (ALS), but despite extensive research into how neurofilaments and peripherin contribute to ALS, their role in this disease is still unidentified.

The neuroimmune system is a system of structures and processes involving the biochemical and electrophysiological interactions between the nervous system and immune system which protect neurons from pathogens. It serves to protect neurons against disease by maintaining selectively permeable barriers, mediating neuroinflammation and wound healing in damaged neurons, and mobilizing host defenses against pathogens.
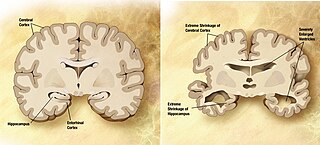
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.
Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, microglia, and oligodendrocytes. In its most extreme form, the proliferation associated with gliosis leads to the formation of a glial scar.
Neurotrophic factors (NTFs) are a family of biomolecules – nearly all of which are peptides or small proteins – that support the growth, survival, and differentiation of both developing and mature neurons. Most NTFs exert their trophic effects on neurons by signaling through tyrosine kinases, usually a receptor tyrosine kinase. In the mature nervous system, they promote neuronal survival, induce synaptic plasticity, and modulate the formation of long-term memories. Neurotrophic factors also promote the initial growth and development of neurons in the central nervous system and peripheral nervous system, and they are capable of regrowing damaged neurons in test tubes and animal models. Some neurotrophic factors are also released by the target tissue in order to guide the growth of developing axons. Most neurotrophic factors belong to one of three families: (1) neurotrophins, (2) glial cell-line derived neurotrophic factor family ligands (GFLs), and (3) neuropoietic cytokines. Each family has its own distinct cell signaling mechanisms, although the cellular responses elicited often do overlap.

Nerve injury is an injury to nervous tissue. There is no single classification system that can describe all the many variations of nerve injuries. In 1941, Seddon introduced a classification of nerve injuries based on three main types of nerve fiber injury and whether there is continuity of the nerve. Usually, however, peripheral nerve injuries are classified in five stages, based on the extent of damage to both the nerve and the surrounding connective tissue, since supporting glial cells may be involved.
Neuroregeneration refers to the regrowth or repair of nervous tissues, cells or cell products. Such mechanisms may include generation of new neurons, glia, axons, myelin, or synapses. Neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS) by the functional mechanisms involved, especially in the extent and speed of repair. When an axon is damaged, the distal segment undergoes Wallerian degeneration, losing its myelin sheath. The proximal segment can either die by apoptosis or undergo the chromatolytic reaction, which is an attempt at repair. In the CNS, synaptic stripping occurs as glial foot processes invade the dead synapse.

Glial scar formation (gliosis) is a reactive cellular process involving astrogliosis that occurs after injury to the central nervous system. As with scarring in other organs and tissues, the glial scar is the body's mechanism to protect and begin the healing process in the nervous system.
Protective autoimmunity is a condition in which cells of the adaptive immune system contribute to maintenance of the functional integrity of a tissue, or facilitate its repair following an insult. The term ‘protective autoimmunity’ was coined by Prof. Michal Schwartz of the Weizmann Institute of Science (Israel), whose pioneering studies were the first to demonstrate that autoimmune T lymphocytes can have a beneficial role in repair, following an injury to the central nervous system (CNS). Most of the studies on the phenomenon of protective autoimmunity were conducted in experimental settings of various CNS pathologies and thus reside within the scientific discipline of neuroimmunology.

Chromatolysis is the dissolution of the Nissl bodies in the cell body of a neuron. It is an induced response of the cell usually triggered by axotomy, ischemia, toxicity to the cell, cell exhaustion, virus infections, and hibernation in lower vertebrates. Neuronal recovery through regeneration can occur after chromatolysis, but most often it is a precursor of apoptosis. The event of chromatolysis is also characterized by a prominent migration of the nucleus towards the periphery of the cell and an increase in the size of the nucleolus, nucleus, and cell body. The term "chromatolysis" was initially used in the 1940s to describe the observed form of cell death characterized by the gradual disintegration of nuclear components; a process which is now called apoptosis. Chromatolysis is still used as a term to distinguish the particular apoptotic process in the neuronal cells, where Nissl substance disintegrates.
Transneuronal degeneration is the death of neurons resulting from the disruption of input from or output to other nearby neurons. It is an active excitotoxic process when a neuron is overstimulated by a neurotransmitter causing the dysfunction of that neuron which drives neighboring neurons into metabolic deficit, resulting in rapid, widespread loss of neurons. This can be either anterograde or retrograde, indicating the direction of the degeneration relative to the original site of damage. There are varying causes for transneuronal degeneration such as brain lesions, disconnection syndromes, respiratory chain deficient neuron interaction, and lobectomies. Although there are different causes, transneuronal degeneration generally results in the same effects to varying degrees. Transneuronal degeneration is thought to be linked to a number of diseases, most notably Huntington's disease and Alzheimer's disease, and researchers recently have been performing experiments with monkeys and rats, monitoring lesions in different parts of the body to study more closely how exactly the process works.
Collapsin response mediator protein family or CRMP family consists of five intracellular phosphoproteins of similar molecular size and high (50–70%) amino acid sequence identity. CRMPs are predominantly expressed in the nervous system during development and play important roles in axon formation from neurites and in growth cone guidance and collapse through their interactions with microtubules. Cleaved forms of CRMPs have also been linked to neuron degeneration after trauma induced injury.

Olfactory ensheathing cells (OECs), also known as olfactory ensheathing glia or olfactory ensheathing glial cells, are a type of macroglia found in the nervous system. They are also known as olfactory Schwann cells, because they ensheath the non-myelinated axons of olfactory neurons in a similar way to which Schwann cells ensheath non-myelinated peripheral neurons. They also share the property of assisting axonal regeneration.
Erythropoietin in neuroprotection is the use of the glycoprotein erythropoietin (Epo) for neuroprotection. Epo controls erythropoiesis, or red blood cell production.
Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. In the central nervous system (CNS), including the brain and spinal cord, microglia are the resident innate immune cells that are activated in response to these cues. The CNS is typically an immunologically privileged site because peripheral immune cells are generally blocked by the blood–brain barrier (BBB), a specialized structure composed of astrocytes and endothelial cells. However, circulating peripheral immune cells may surpass a compromised BBB and encounter neurons and glial cells expressing major histocompatibility complex molecules, perpetuating the immune response. Although the response is initiated to protect the central nervous system from the infectious agent, the effect may be toxic and widespread inflammation as well as further migration of leukocytes through the blood–brain barrier.
Preferential motor reinnervation (PMR) refers to the tendency of a regenerating axon in the peripheral nervous system (PNS) to reinnervate a motor pathway as opposed to a somatosensory pathway. PMR affects how nerves regenerate and reinnervate within the PNS after surgical procedures or traumatic injuries. It is important to understand in order to further develop axonal regrowth surgical techniques. Further research of preferential motor reinnervation will lead to a better understanding of peripheral nervous system function in the human body regarding cell roles and abilities.










