
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from Ancient Greek βρῶμος (bromos) 'stench', referring to its sharp and pungent smell.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.
A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group.
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.

The mass number (symbol A, from the German word: Atomgewicht, "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number A is identical with the baryon number B of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number Z gives the number of neutrons (N) in the nucleus: N = A − Z.
In organic chemistry, free-radical addition is an addition reaction which involves free radicals. Radical additions are known for a variety of unsaturated substrates, both olefinic or aromatic and with or without heteroatoms.

Halomethane compounds are derivatives of methane with one or more of the hydrogen atoms replaced with halogen atoms. Halomethanes are both naturally occurring, especially in marine environments, and human-made, most notably as refrigerants, solvents, propellants, and fumigants. Many, including the chlorofluorocarbons, have attracted wide attention because they become active when exposed to ultraviolet light found at high altitudes and destroy the Earth's protective ozone layer.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
In chemistry the descriptor vicinal, abbreviated vic, is a descriptor that identifies two functional groups as bonded to two adjacent carbon atoms. It may arise from vicinal difunctionalization.
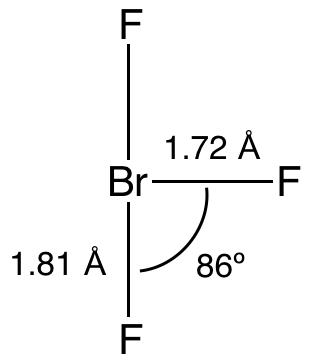
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.

A halonium ion is any onium ion containing a halogen atom carrying a positive charge. This cation has the general structure R−+X−R′ where X is any halogen and no restrictions on R, this structure can be cyclic or an open chain molecular structure. Halonium ions formed from fluorine, chlorine, bromine, and iodine are called fluoronium, chloronium, bromonium, and iodonium, respectively. The 3-membered cyclic variety commonly proposed as intermediates in electrophilic halogenation may be called haliranium ions, using the Hantzsch-Widman nomenclature system.
In stereochemistry, topicity is the stereochemical relationship between substituents and the structure to which they are attached. Depending on the relationship, such groups can be heterotopic, homotopic, enantiotopic, or diastereotopic.

Gold(III) bromide is a dark-red to black crystalline solid. It has the empirical formula AuBr3, but exists as a dimer with the molecular formula Au2Br6 in which two gold atoms are bridged by two bromine atoms. It is commonly referred to as gold(III) bromide, gold tribromide, and rarely but traditionally auric bromide, and sometimes as digold hexabromide. The analogous copper or silver tribromides do not exist.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.
The Fleming–Tamao oxidation, or Tamao–Kumada–Fleming oxidation, converts a carbon–silicon bond to a carbon–oxygen bond with a peroxy acid or hydrogen peroxide. Fleming–Tamao oxidation refers to two slightly different conditions developed concurrently in the early 1980s by the Kohei Tamao and Ian Fleming research groups.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
Mercury(I) bromide or mercurous bromide is the chemical compound composed of mercury and bromine with the formula Hg2Br2. It changes color from white to yellow when heated and fluoresces a salmon color when exposed to ultraviolet light. It has applications in acousto-optical devices.
Manganese(II) bromide is the chemical compound composed of manganese and bromine with the formula MnBr2.

1,1,2,2-Tetrabromoethane, or simply tetrabromoethane (TBE), is a halogenated hydrocarbon, chemical formula C2H2Br4. Although three bromine atoms may bind to one of the carbon atoms creating 1,1,1,2-tetrabromoethane this is not thermodynamically favorable, so in practice tetrabromoethane is equal to 1,1,2,2-tetrabromoethane, where each carbon atom binds two bromine atoms.

Dibromine pentoxide is the chemical compound composed of bromine and oxygen with the formula Br2O5. It is a colorless solid that is stable below −20 °C. It has the structure O2Br−O−BrO2, the Br−O−Br bond is bent with bond angle 121.2°. Each BrO3 group is pyramidal with the bromine atom at the apex.













