Cyclohexa-1,3-diene (also known as Benzane) is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). A naturally occurring derivative of cyclohexa-1,3-diene is terpinene, a component of pine oil.

Polypyridine complexes are coordination complexes containing polypyridine ligands, such as 2,2'-bipyridine, 1,10-phenanthroline, or 2,2';6'2"-terpyridine.

Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry.

Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry.
The transition metal ruthenium forms several compounds, with oxidation states of ruthenium ranging from 0 to +8, and −2. The properties of ruthenium and osmium compounds are often similar. The +2, +3, and +4 states are the most common. The most prevalent precursor is ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.
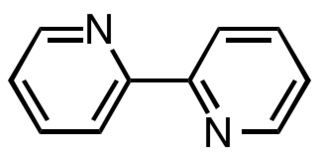
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications.

Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.

Tris(bipyridine)ruthenium(II) chloride is the chloride salt coordination complex with the formula [Ru(bpy)3]2+ 2Cl−. This polypyridine complex is a red crystalline salt obtained as the hexahydrate, although all of the properties of interest are in the cation [Ru(bpy)3]2+, which has received much attention because of its distinctive optical properties. The chlorides can be replaced with other anions, such as PF6−.

Electrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes.

(Cymene)ruthenium dichloride dimer is the organometallic compound with the formula [(cymene)RuCl2]2. This red-coloured, diamagnetic solid is a reagent in organometallic chemistry and homogeneous catalysis. The complex is structurally similar to (benzene)ruthenium dichloride dimer.

Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used in homogeneous catalysis.
Photochemical reduction of carbon dioxide harnesses solar energy to convert CO2 into higher-energy products. Environmental interest in producing artificial systems is motivated by recognition that CO2 is a greenhouse gas. The process has not been commercialized.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
DNA-binding metallo-intercalators are positively charged, planar, polycyclic, aromatic compounds that unwind the DNA double helix and insert themselves between DNA base pairs. Metallo-intercalators insert themselves between two intact base pairs without expelling or replacing the original nitrogenous bases; the hydrogen bonds between the nitrogenous bases at the site of intercalation remain unbroken. In addition to π-stacking between the aromatic regions of the intercalator and the nitrogenous bases of DNA, intercalation is stabilized by van der Waals, hydrophobic, electrostatic, and entropic interactions. This ability to bind to specific DNA base pairs allows for potential therapeutic applications of metallo-intercalators.
Photo-Induced Cross-Linking of Unmodified Proteins (PICUP) is a protein cross-linking method by visible light irradiation of a photocatalyst in the presence of an electron acceptor and the protein of interest. Irradiation results in a highly reactive protein radical that forms a covalent bond between the amino acid side chains of the proteins to be linked. Cross-linking methods developed prior to PICUP, including the use of physical, oxidative, and chemical cross-linkers, often require more time and result in protein byproducts. In addition, the cross-linked protein yield is very low due to the multifunctionality of the cross-linking reagents.

(Benzene)ruthenium dichloride dimer is the organoruthenium compound with the formula [(C6H6)RuCl2]2. This red-coloured, diamagnetic solid is a reagent in organometallic chemistry and homogeneous catalysis.

cis-Dichlorobis(bipyridine)ruthenium(II) is the coordination complex with the formula RuCl2(bipy)2, where bipy is 2,2'-bipyridine. It is a dark green diamagnetic solid that is a precursor to many other complexes of ruthenium, mainly by substitution of the two chloride ligands. The compound has been crystallized as diverse hydrates.
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.
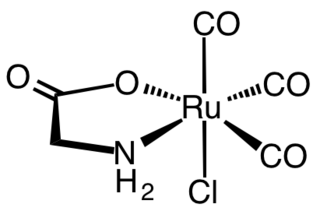
Tricarbonylchloroglycinatoruthenium(II) is an organoruthenium complex with the formula RuCl(H2NCH2CO2)(CO)3. A yellow solid, it is an amino acid complex consisting of an octahedral complex with three carbonyls, chloride, and bidentate glycinate ligands. The CO ligands are arranged in a facial geometry. The complex is prepared by treating dichlororuthenium tricarbonyl dimer with sodium glycinate. The complex has attracted attention as a CO-releasing molecule ("CORM").













