Related Research Articles

Metabolism is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks of proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary metabolism.

Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is deoxyribose, a variant of ribose, the polymer is DNA.
Nitrogen fixation is a chemical process by which molecular dinitrogen is converted into ammonia. It occurs both biologically and abiologically in chemical industries. Biological nitrogen fixation or diazotrophy is catalyzed by enzymes called nitrogenases. These enzyme complexes are encoded by the Nif genes and contain iron, often with a second metal.

Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. Biophysical research shares significant overlap with biochemistry, molecular biology, physical chemistry, physiology, nanotechnology, bioengineering, computational biology, biomechanics, developmental biology and systems biology.

Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.
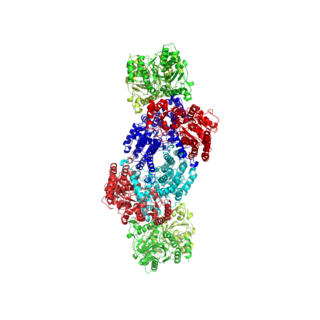
Nitrogenases are enzymes (EC 1.18.6.1EC 1.19.6.1) that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase.
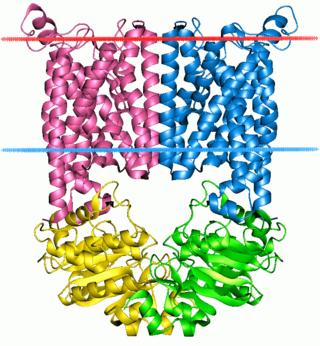
The ABC transporters, ATP synthase (ATP)-binding cassette transporters are a transport system superfamily that is one of the largest and possibly one of the oldest gene families. It is represented in all extant phyla, from prokaryotes to humans. ABC transporters belong to translocases.

Azotobacter is a genus of usually motile, oval or spherical bacteria that form thick-walled cysts and may produce large quantities of capsular slime. They are aerobic, free-living soil microbes that play an important role in the nitrogen cycle in nature, binding atmospheric nitrogen, which is inaccessible to plants, and releasing it in the form of ammonium ions into the soil. In addition to being a model organism for studying diazotrophs, it is used by humans for the production of biofertilizers, food additives, and some biopolymers. The first representative of the genus, Azotobacter chroococcum, was discovered and described in 1901 by Dutch microbiologist and botanist Martinus Beijerinck. Azotobacter species are Gram-negative bacteria found in neutral and alkaline soils, in water, and in association with some plants.
Azotobacter vinelandii is Gram-negative diazotroph that can fix nitrogen while grown aerobically. These bacteria are easily cultured and grown.
Benoît Roux is an Amgen Professor of Biochemistry and Molecular Biophysics at the University of Chicago. He has previously taught at University of Montreal and Weill Medical College of Cornell University. Benoît Roux was a recipient of the 1998 Rutherford Memorial Medal in Chemistry, awarded by the Royal Society of Canada.

Molecular biophysics is a rapidly evolving interdisciplinary area of research that combines concepts in physics, chemistry, engineering, mathematics and biology. It seeks to understand biomolecular systems and explain biological function in terms of molecular structure, structural organization, and dynamic behaviour at various levels of complexity. This discipline covers topics such as the measurement of molecular forces, molecular associations, allosteric interactions, Brownian motion, and cable theory. Additional areas of study can be found on Outline of Biophysics. The discipline has required development of specialized equipment and procedures capable of imaging and manipulating minute living structures, as well as novel experimental approaches.
The following outline is provided as an overview of and topical guide to biophysics:

The Nif regulon is a set of seven operons used to regulate nitrogen fixation in the coliform bacterium Klebsiella pneumoniae under anaerobic and microaerophilic conditions. It includes 17 nif genes, and is situated between the his and the Shi-A operon of the bacterium.

In molecular biology, the term macromolecular assembly (MA) refers to massive chemical structures such as viruses and non-biologic nanoparticles, cellular organelles and membranes and ribosomes, etc. that are complex mixtures of polypeptide, polynucleotide, polysaccharide or other polymeric macromolecules. They are generally of more than one of these types, and the mixtures are defined spatially, and with regard to their underlying chemical composition and structure. Macromolecules are found in living and nonliving things, and are composed of many hundreds or thousands of atoms held together by covalent bonds; they are often characterized by repeating units. Assemblies of these can likewise be biologic or non-biologic, though the MA term is more commonly applied in biology, and the term supramolecular assembly is more often applied in non-biologic contexts. MAs of macromolecules are held in their defined forms by non-covalent intermolecular interactions, and can be in either non-repeating structures, or in repeating linear, circular, spiral, or other patterns. The process by which MAs are formed has been termed molecular self-assembly, a term especially applied in non-biologic contexts. A wide variety of physical/biophysical, chemical/biochemical, and computational methods exist for the study of MA; given the scale of MAs, efforts to elaborate their composition and structure and discern mechanisms underlying their functions are at the forefront of modern structure science.
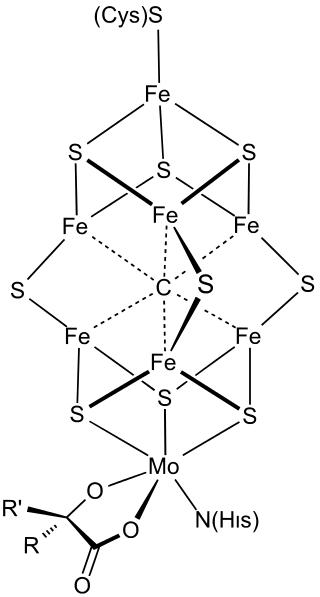
FeMoco (FeMo cofactor) is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Because it contains iron and molybdenum, the cofactor is called FeMoco. Its stoichiometry is Fe7MoS9C.
Kalpathy Ramaier Katchap Easwaran is an Indian molecular biophysicist, academic and a former Astra Chair Professor and chairman of the department of molecular biophysics of the Indian Institute of Science. He is known for his contributions in the development of anti-fungal drugs and for his researches on ionophores and ion-transport across membranes. He is an elected fellow of the Indian National Science Academy and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1984, for his contributions to biological sciences.
Jue Chen is a Chinese-born American structural biologist and biochemist. She is the William E. Ford professor of biochemistry and head of the Laboratory of Membrane Biology and Biophysics at the Rockefeller University and a Howard Hughes Medical Institute investigator. Her research focuses on elucidating the structure and function of ATP-binding cassette (ABC) transporters.
Robert Michael Stroud is a British biophysicist best known for his contributions to structural biology as means of determining the function of proteins, enzymes and integral membrane proteins. He was a professor of Chemistry at Caltech in the early 1970s and professor of Biochemistry and Biophysics, and of Pharmaceutical Chemistry at the University of California in San Francisco since 1976. He was elected to the National Academy of Sciences in 2003.
Alexander Glazer was a Polish-born American professor of the Graduate School in the Department of Molecular and Cell Biology at the University of California, Berkeley. He had a passion for protein chemistry and structure function relationships. He also had a longstanding interest in light-harvesting complexes in cyanobacteria and red algae called phycobilisomes. He had also spent more than 10 years working on the human genome project where he has investigated methods for DNA detection and sequencing which most notably includes the development of fluorescent reagents involved in cell labeling. Most recently, he had focused his studies on issues in environmental sciences. He died on July 18, 2021, in Orinda, California, at the age of 86.
Perry William Wilson was an American microbiologist and biochemist. He gained a scientific reputation as an outstanding pioneer in transforming the science of biological nitrogen fixation. His research helped to transform a mainly descriptive science into a more quantitative and analytic science based on biochemistry and statistical methods and control in bacteriology. He was called the "dean of biological nitrogen fixation".
References
- ↑ Douglas C. Rees publications from Europe PubMed Central
- ↑ Career data from Pamela Kalte, et al., American Men and Women of Science, Thomson Gale 2004
- ↑ Rees, Douglas C. (2004). "Preface by Douglas C. Rees". Annual Review of Biophysics and Biomolecular Structure. 33. doi:10.1146/annurev.bb.33.051304.100011.
- ↑ Rees, Douglas C. (2012). "Preface". Annual Review of Biophysics. 41. doi:10.1146/annurev-bb-41-050712-100001.