
Analcime (; from Ancient Greek ἀνάλκιμος (análkimos) 'not strong') or analcite is a white, gray, or colorless tectosilicate mineral. Analcime consists of hydrated sodium aluminium silicate in cubic crystalline form. Its chemical formula is NaAlSi2O6·H2O. Minor amounts of potassium and calcium substitute for sodium. A silver-bearing synthetic variety also exists (Ag-analcite). Analcime is usually classified as a zeolite mineral, but structurally and chemically it is more similar to the feldspathoids. Analcime isn't classified as an isometric crystal, as although the crystal structure appears to be isometric, it is usually off only by a fraction of an angle. However, there are truly isometric samples of the mineral, which makes its classification even more difficult. Due to the differences between the samples being too slight, there's no merit from having multiple species names, so as a result analcime is a common example for minerals occurring in multiple crystal systems and space groups. It was first described by French geologist Déodat de Dolomieu, who called it zéolithe dure, meaning hard zeolite. It was found in lava in Cyclops, Italy. The mineral is IMA approved, and had been grandfathered, meaning the name analcime is believed to refer to a valid species til this day.

In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

Strontianite (SrCO3) is an important raw material for the extraction of strontium. It is a rare carbonate mineral and one of only a few strontium minerals. It is a member of the aragonite group.
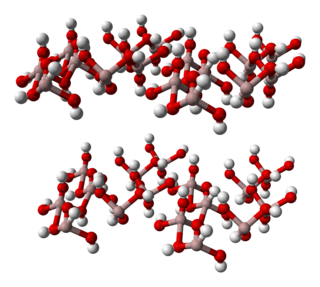
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic and acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina, the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water.

Gibbsite, Al(OH)3, is one of the mineral forms of aluminium hydroxide. It is often designated as γ-Al(OH)3 (but sometimes as α-Al(OH)3.). It is also sometimes called hydrargillite (or hydrargyllite).

Sodalite is a tectosilicate mineral with the formula Na
8(Al
6Si
6O
24)Cl
2, with royal blue varieties widely used as an ornamental gemstone. Although massive sodalite samples are opaque, crystals are usually transparent to translucent. Sodalite is a member of the sodalite group with hauyne, nosean, lazurite and tugtupite.

The mineral marcasite, sometimes called "white iron pyrite", is iron sulfide (FeS2) with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both structures contain the disulfide S22− ion, having a short bonding distance between the sulfur atoms. The structures differ in how these di-anions are arranged around the Fe2+ cations. Marcasite is lighter and more brittle than pyrite. Specimens of marcasite often crumble and break up due to the unstable crystal structure.

Dickite is a phyllosilicate clay mineral named after the metallurgical chemist Allan Brugh Dick, who first described it. It is chemically composed of 20.90% aluminium, 21.76% silicon, 1.56% hydrogen and 55.78% oxygen. It has the same composition as kaolinite, nacrite, and halloysite, but with a different crystal structure (polymorph). Dickite sometimes contains impurities such as titanium, iron, magnesium, calcium, sodium and potassium.

Halotrichite, also known as feather alum, is a highly hydrated sulfate of aluminium and iron. Its chemical formula is FeAl2(SO4)4·22H2O. It forms fibrous monoclinic crystals. The crystals are water-soluble.

Ajoite is a hydrated sodium potassium copper aluminium silicate hydroxide mineral. Ajoite has the chemical formula (Na,K)Cu7AlSi9O24(OH)6·3H2O, and minor Mn, Fe and Ca are usually also present in the structure. Ajoite is used as a minor ore of copper.
Cleusonite is a member of the crichtonite group of minerals with the chemical formula (Pb,Sr)(U4+
,U6+
)(Fe2+
,Zn)
2(Ti,Fe2+
,Fe3+
)
18(O,OH)
38. This group of minerals contains approximately thirteen complex metal titanates. The structures of minerals of this group is complicated by frequent fine-scale twinning and metamictization due to radioactive elements. The crichtonite group consists of members of related mineral species of the type A{BC2D6E12}O38 which are characterized by their predominant cations (as seen in crichtonite (Sr), senaite (Pb), davidite (REE + U), landauite (Na), loveringite (Ca), lindsleyite (Ba), and mathiasite (K).
Clearcreekite is a carbonate mineral, polymorphous with peterbaylissite. The chemical formula of clearcreekite is Hg1+3CO3(OH)∙2H2O. It has a pale greenish yellow color and streak with tabular subhedral crystals and good cleavage on {001}. It is transparent with vitreous luster and uneven fracture. Its density (calculated from the idealized formula) is 6.96 g/cm3. The mineral is monoclinic with the space group P2/c. Clearcreekite is an extremely rare mineral from the Clear Creek mercury mine, New Idria district, San Benito County, California. It was probably formed after the alteration of other mercury minerals such as cinnabar. The mineral is named after the locality where it was found.

Quintinite is a carbonate mineral with the chemical formula Mg4Al2(OH)12CO3⋅3H2O.

Cookeite is a mineral species of the silicate group and the phyllosilicate subgroup, part of the chlorite family, with the formula LiAl4(Si3Al)O10(OH)8. This soft, low-density mineral of variable color has a crystalline structure made up of alternating layers LiAl2(OH)6 and Al2O4(OH)2Si8O12 having several polytypes. Cookeite is often found as a product of hydrothermal alteration of silicates in pegmatites. It forms at relatively low temperatures (below 200°C) and variable pressures.

Serandite is a mineral with formula Na(Mn2+,Ca)2Si3O8(OH). The mineral was discovered in Guinea in 1931 and named for J. M. Sérand. Serandite is generally red, brown, black or colorless. The correct name lacks an accent.

Guyanaite (CrOOH) is a chromium oxide mineral that forms as an intergrowth with other chromium oxide minerals known as bracewellite (CrOOH) and grimaldiite (CrOOH) as well as eskolaite (Cr2O3) which in early findings were nearly indistinguishable from one another. These oxides formed so closely as intergrowths with one another that they were initially, and erroneously, identified as a single definite mineral previously known as merumite. Because of its complex history and the previously undiscovered nature of these chromium oxide polymorphs, the relevance of any information found in many early experiments involving the mineral formerly known as merumite in regard to guyanaite is unknown and it is implied that in any further reference of merumite it will have been composed of a mineral assemblage including guyanaite. The rare occurrence and complexity from intergrowth of naturally occurring guyanaite hinders experimental work, leading to laboratory synthesized samples which help to better experiment with the minerals.
Johnsenite-(Ce) is a very rare mineral of the eudialyte group, with the chemical formula Na12(Ce,La,Sr,Ca,[ ])3Ca6Mn3Zr3WSi(Si9O27)2(Si3O9)2(CO3)O(OH,Cl)2. The original formula was extended to show the presence of both the cyclic silicate groups and silicon at the M4 site, according to the nomenclature of the eudialyte group. It is the third eudialyte-group mineral with essential tungsten, and second with essential rare earth elements. In fact, some niobium substitutes for tungsten in johnsenite-(Ce). Other characteristic feature is the presence of essential carbonate group, shared with carbokentbrooksite, golyshevite, mogovidite and zirsilite-(Ce).
Sheldrickite is a sodium calcium carbonate fluoride mineral, named in honor of George M. Sheldrick, former Professor of Crystallography at the University of Göttingen in Germany. Sheldrick is the creator of SHELLX computer program widely used for the analysis of crystal structures. Determination of the structure of this mineral required the software's capability of handling twinned crystals.
Cattiite is a phosphate mineral. The mineral was first found in a veins of dolomite carbonatites veins at the bottom of the Zhelezny (Iron) Mine in the Kovdor massif, Kola Peninsula, Russia. Cattiite was tentatively identified as Mg3(PO4)2•22H2O, which as a high hydrate magnesium orthophosphate. Later structural studies, revealed the existence of two polytypes named Mg3(PO4)2•22H2O-1A1 and Mg3(PO4)2•22H2O-1A2.

Paravauxite is a rare mineral that was named in 1922. Its name is a portmanteau word made by blending the Greek word for near and vauxite due to the chemical relationship to vauxite. It was approved by the IMA, and was first described in 1959. It is now grandfathered, meaning it is probably to remain a species.















