
Adhesive, also known as glue, cement, mucilage, or lauren, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
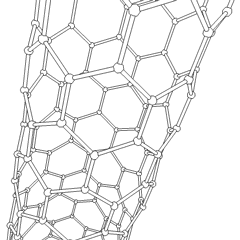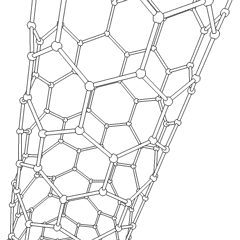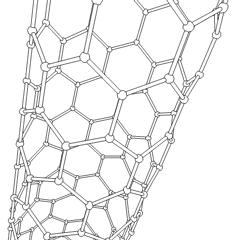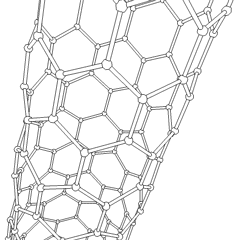
Carbon nanotubes (CNTs) are tubes made of carbon with diameters typically measured in nanometers.

Geckos are small, mostly carnivorous lizards that have a wide distribution, found on every continent except Antarctica.

In molecular physics, the van der Waals force, named after Dutch physicist Johannes Diderik van der Waals, is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules.
In biology, setae are any of a number of different bristle- or hair-like structures on living organisms.

Synthetic setae emulate the setae found on the toes of a gecko and scientific research in this area is driven towards the development of dry adhesives. Geckos have no difficulty mastering vertical walls and are apparently capable of adhering themselves to just about any surface. The five-toed feet of a gecko are covered with elastic hairs called setae and the ends of these hairs are split into nanoscale structures called spatulae. The sheer abundance and proximity to the surface of these spatulae make it sufficient for van der Waals forces alone to provide the required adhesive strength. Following the discovery of the gecko's adhesion mechanism in 2002, which is based on van der Waals forces, biomimetic adhesives have become the topic of a major research effort. These developments are poised to yield families of novel adhesive materials with superior properties which are likely to find uses in industries ranging from defense and nanotechnology to healthcare and sport.
An inorganic nanotube is a cylindrical molecule often composed of metal oxides, or group III-Nitrides and morphologically similar to a carbon nanotube. Inorganic nanotubes have been observed to occur naturally in some mineral deposits.

Carbon nanotubes (CNTs) are cylinders of one or more layers of graphene (lattice). Diameters of single-walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes (MWNTs) are typically 0.8 to 2 nm and 5 to 20 nm, respectively, although MWNT diameters can exceed 100 nm. CNT lengths range from less than 100 nm to 0.5 m.
CD2 is a cell adhesion molecule found on the surface of T cells and natural killer (NK) cells. It has also been called T-cell surface antigen T11/Leu-5, LFA-2, LFA-3 receptor, erythrocyte receptor and rosette receptor.
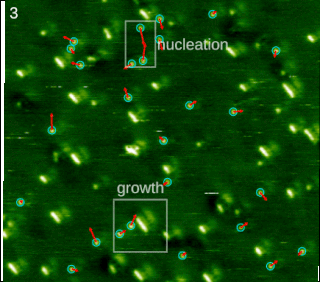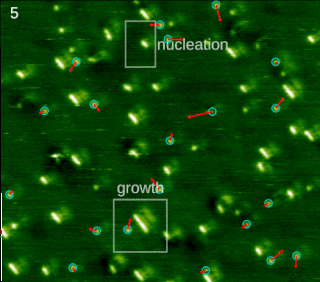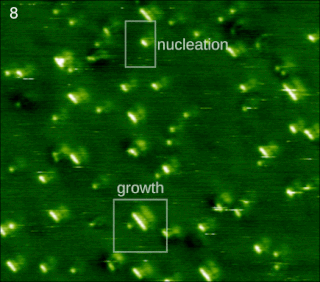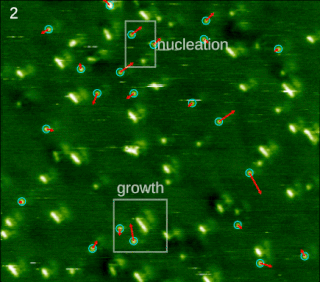
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly. These are intramolecular self-assembly and intermolecular self-assembly. Commonly, the term molecular self-assembly refers to intermolecular self-assembly, while the intramolecular analog is more commonly called folding.

Adhesive tape refers to any one of a variety of combinations of backing materials coated with an adhesive. Different backing materials and adhesives can be used depending on the intended use.
Kim D. Janda is an American chemist who studies on medicinal chemistry, molecular biology, immunology and neuropharmacology.

The optical properties of carbon nanotubes are highly relevant for materials science. The way those materials interact with electromagnetic radiation is unique in many respects, as evidenced by their peculiar absorption, photoluminescence (fluorescence), and Raman spectra.
Graham R. Fleming is a Professor of Chemistry at the University of California, Berkeley and member of the Kavli Energy NanoScience Institute based at UCB.
A catch bond is a type of noncovalent bond whose dissociation lifetime increases with tensile force applied to the bond. Normally, bond lifetimes are expected to diminish with force. In the case of catch bonds, the lifetime of the bond actually increases up to a maximum before it decreases like in a normal bond. Catch bonds work in a way that is conceptually similar to that of a Chinese finger trap. While catch bonds are strengthened by an increase in force, the force increase is not necessary for the bond to work. Catch bonds were suspected for many years to play a role in the rolling of leukocytes, being strong enough to roll in presence of high forces caused by high shear stresses, while avoiding getting stuck in capillaries where the fluid flow, and therefore shear stress, is low. The existence of catch bonds was debated for many years until strong evidence of their existence was found in bacteria. Definite proof of their existence came shortly thereafter in leukocytes.

The feet of geckos have a number of specializations. Their surfaces can adhere to any type of material with the exception of Teflon (PTFE). This phenomenon can be explained with three elements:
Penta-graphene is a hypothetical carbon allotrope composed entirely of carbon pentagons and resembling the Cairo pentagonal tiling. Penta-graphene was proposed in 2014 on the basis of analyses and simulations. Further calculations showed that it is unstable in its pure form, but can be stabilized by hydrogenation. Owing to its atomic configuration, penta-graphene has an unusually negative Poisson’s ratio and very high ideal strength believed to exceed that of a similar material, graphene.

Arthropods, including insects and spiders, make use of smooth adhesive pads as well as hairy pads for climbing and locomotion along non-horizontal surfaces. Both types of pads in insects make use of liquid secretions and are considered 'wet'. Dry adhesive mechanisms primarily rely on van der Waals’ forces and are also used by organisms other than insects. The fluid provides capillary and viscous adhesion and appears to be present in all insect adhesive pads. Little is known about the chemical properties of the adhesive fluids and the ultrastructure of the fluid producing cells is currently not extensively studied. Additionally, both hairy and smooth types of adhesion have evolved separately numerous times in insects. Few comparative studies between the two types of adhesion mechanisms have been done and there is a lack of information regarding the forces that can be supported by these systems in insects. Additionally, tree frogs and some mammals such as the arboreal possum and bats also make use of smooth adhesive pads. The use of adhesive pads for locomotion across non-horizontal surfaces is a trait that evolved separately in different species, making it an example of convergent evolution. The power of adhesion allows these organisms to be able to climb on almost any substance.
Self-cleaning surfaces are a class of materials with the inherent ability to remove any debris or bacteria from their surfaces in a variety of ways. The self-cleaning functionality of these surfaces are commonly inspired by natural phenomena observed in lotus leaves, gecko feet, and water striders to name a few. The majority of self-cleaning surfaces can be placed into three categories: 1) superhydrophobic, 2) superhydrophilic, and 3) photocatalytic.

Nano tape, also called gecko tape; marketed under the name Alien Tape, is a synthetic adhesive tape consisting of arrays of carbon nanotubes transferred onto a backing material of flexible polymer tape. These arrays are called synthetic setae and mimic the nanostructures found on the toes of a gecko; this is an example of biomimicry. The adhesion is achieved not with chemical adhesives, but via van der Waals forces, which are weak electric forces generated between two atoms or molecules that are very close to each other.














