
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name Piper, which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins.

The Feist–Benary synthesis is an organic reaction between α-halogen ketones and β-dicarbonyl compounds to produce substituted furan compounds. This condensation reaction is catalyzed by amines such as ammonia and pyridine. The first step in the ring synthesis is related to the Knoevenagel condensation. In the second step the enolate displaces an alkyl halogen in a nucleophilic aliphatic substitution.

Lupinine is a quinolizidine alkaloid present in the genus Lupinus of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of Lupinus plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of Lupinus.
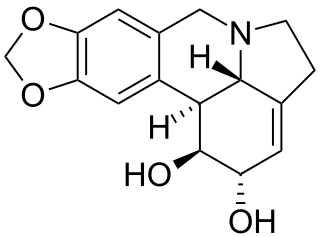
Lycorine is a toxic crystalline alkaloid found in various Amaryllidaceae species, such as the cultivated bush lily, surprise lilies (Lycoris), and daffodils (Narcissus). It may be highly poisonous, or even lethal, when ingested in certain quantities. Regardless, it is sometimes used medicinally, a reason why some groups may harvest the very popular Clivia miniata.
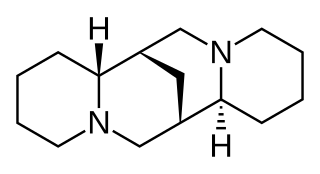
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in Lupinus mutabilis, and is thought to chelate the bivalent cations calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughan Williams classification of antiarrhythmic drugs.

Mesembrine is an alkaloid present in Sceletium tortuosum (kanna). It has been shown to act as a serotonin reuptake inhibitor (Ki = 1.4 nM), and more recently, has also been found to behave as a weak inhibitor of the enzyme phosphodiesterase 4 (PDE4) (Ki = 7,800 nM). Still more recently, in an in vitro study published in 2015, scientist concluded that "a high-mesembrine Sceletium extract exerts its anti-depressant effect by acting primarily as monoamine releasing agent, rather than as selective serotonin-reuptake inhibitor." As such, mesembrine likely plays a dominant role in the antidepressant effects of kanna. The levorotatory isomer, (−)-mesembrine, is the natural form.

3,4-Dimethoxyphenethylamine (DMPEA) is a chemical compound of the phenethylamine class. It is an analogue of the major human neurotransmitter dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine.

5-Bromo-DMT (5-bromo-N,N-dimethyltryptamine) is a psychedelic brominated indole alkaloid found in the sponges Smenospongia aurea and Smenospongia echina, as well as in Verongula rigida alongside 5,6-Dibromo-DMT and seven other alkaloids. It is the 5-bromo derivative of DMT, a psychedelic found in many plants and animals.
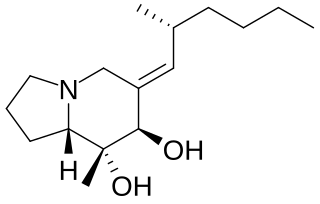
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates. It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D. It has been tested on mice and found to be five times more potent than the former version. It has been produced synthetically through a variety of different routes.

Larry E. Overman is Distinguished Professor of Chemistry at the University of California, Irvine. He was born in Chicago in 1943. Overman obtained a B.A. degree from Earlham College in 1965, and he completed his Ph.D. in chemistry from the University of Wisconsin–Madison in 1969, under Howard Whitlock Jr. Professor Overman is a member of the United States National Academy of Sciences and the American Academy of Arts and Sciences. He was the recipient of the Arthur C. Cope Award in 2003, and he was awarded the Tetrahedron Prize for Creativity in Organic Chemistry for 2008.
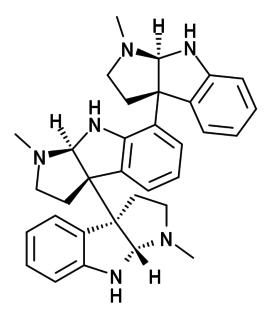
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata, although it is also found in Psychotria lyciiflora and probably other species in this family,
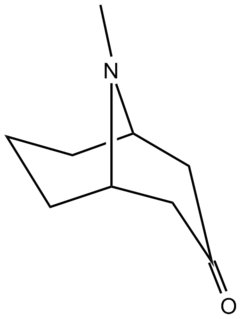
Pseudopelletierine is the main alkaloid derived from the root-bark of the pomegranate tree, along with at least three other alkaloids: pelletierine, isopelletierine, and methylpelleteirine (C9H17ON), which yield 1.8, 0.52, 0.01, and 0.20 grams per kilogram of raw bark.

Indole is an aromatic heterocyclic organic compound with formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.
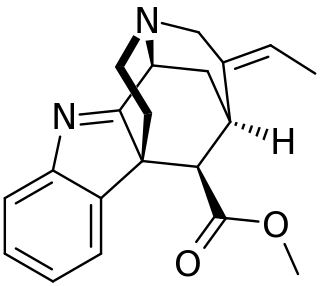
Strictamine is an alkaloid isolated from Alstonia scholaris.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.
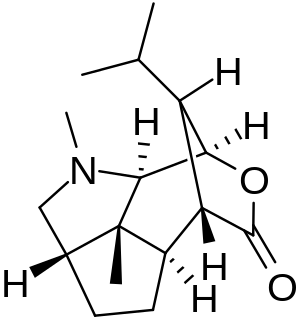
Dendrobine is an alkaloid found in Dendrobium nobile at an average of 0.5% by weight. It is a colorless solid at room temperature. It is related to the picrotoxin family of natural products. When given a fatal dose, death is usually caused by convulsions. It possesses a molecular structure that attracted interest in its total synthesis by organic chemists.

Setoclavine is an ergot alkaloid.
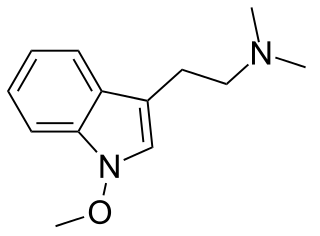
Lespedamine is an indole alkaloid and substituted tryptamine present in the plant Lespedeza bicolor. The alkaloid bears a close structural resemblance to the psychedelic alkaloid dimethyltryptamine and was speculated to have psychoactivity by Alexander Shulgin. No reports on lespedamine's biological activity have been published.

Alstonia congensis, is a tree within the Apocynaceae family and one of two African species within the Alstonia genus, the other being the Alstonia boonei De Wild. Both have similar morphological characteristics.




















