Related Research Articles

In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids, and nucleic acids, as well as small molecules such as vitamins and hormones. A more general name for this class of material is biological materials. Biomolecules are an important element of living organisms, those biomolecules are often endogenous, produced within the organism but organisms usually need exogenous biomolecules, for example certain nutrients, to survive.
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the target of the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample. An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit.
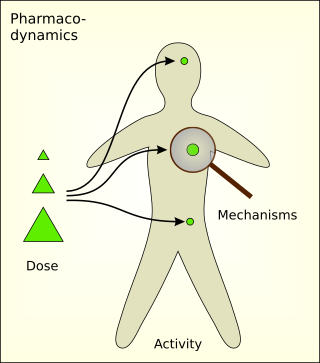
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs. The effects can include those manifested within animals, microorganisms, or combinations of organisms.
In a variety of contexts, exogeny or exogeneity is the fact of an action or object originating externally. It contrasts with endogeneity or endogeny, the fact of being influenced within a system.

A molecular lesion or point lesion is damage to the structure of a biological molecule such as DNA, RNA, or protein. This damage may result in the reduction or absence of normal function, and in rare cases the gain of a new function. Lesions in DNA may consist of breaks or other changes in chemical structure of the helix, ultimately preventing transcription. Meanwhile, lesions in proteins consist of both broken bonds and improper folding of the amino acid chain. While many nucleic acid lesions are general across DNA and RNA, some are specific to one, such as thymine dimers being found exclusively in DNA. Several cellular repair mechanisms exist, ranging from global to specific, in order to prevent lasting damage resulting from lesions.

Nonylphenols are a family of closely related organic compounds composed of phenol bearing a 9 carbon-tail. Nonylphenols can come in numerous structures, all of which may be considered alkylphenols. They are used in manufacturing antioxidants, lubricating oil additives, laundry and dish detergents, emulsifiers, and solubilizers. They are used extensively in epoxy formulation in North America but its use has been phased out in Europe. These compounds are also precursors to the commercially important non-ionic surfactants alkylphenol ethoxylates and nonylphenol ethoxylates, which are used in detergents, paints, pesticides, personal care products, and plastics. Nonylphenol has attracted attention due to its prevalence in the environment and its potential role as an endocrine disruptor and xenoestrogen, due to its ability to act with estrogen-like activity. The estrogenicity and biodegradation heavily depends on the branching of the nonyl sidechain. Nonylphenol has been found to act as an agonist of the GPER (GPR30).
Antigen processing, or the cytosolic pathway, is an immunological process that prepares antigens for presentation to special cells of the immune system called T lymphocytes. It is considered to be a stage of antigen presentation pathways. This process involves two distinct pathways for processing of antigens from an organism's own (self) proteins or intracellular pathogens, or from phagocytosed pathogens ; subsequent presentation of these antigens on class I or class II major histocompatibility complex (MHC) molecules is dependent on which pathway is used. Both MHC class I and II are required to bind antigens before they are stably expressed on a cell surface. MHC I antigen presentation typically involves the endogenous pathway of antigen processing, and MHC II antigen presentation involves the exogenous pathway of antigen processing. Cross-presentation involves parts of the exogenous and the endogenous pathways but ultimately involves the latter portion of the endogenous pathway.
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estrogenic effects on a living organism even though they differ chemically from the estrogenic substances produced internally by the endocrine system of any organism. Natural xenoestrogens include phytoestrogens which are plant-derived xenoestrogens. Because the primary route of exposure to these compounds is by consumption of phytoestrogenic plants, they are sometimes called "dietary estrogens". Mycoestrogens, estrogenic substances from fungi, are another type of xenoestrogen that are also considered mycotoxins.

Endogenous retroviruses (ERVs) are endogenous viral elements in the genome that closely resemble and can be derived from retroviruses. They are abundant in the genomes of jawed vertebrates, and they comprise up to 5–8% of the human genome.

Exogenous DNA is DNA originating outside the organism of concern or study. Exogenous DNA can be found naturally in the form of partially degraded fragments left over from dead cells. These DNA fragments may then become integrated into the chromosomes of nearby bacterial cells to undergo mutagenesis. This process of altering bacteria is known as transformation. Bacteria may also undergo artificial transformation through chemical and biological processes. The introduction of exogenous DNA into eukaryotic cells is known as transfection. Exogenous DNA can also be artificially inserted into the genome, which revolutionized the process of genetic modification in animals. By microinjecting an artificial transgene into the nucleus of an animal embryo, the exogenous DNA is allowed to merge the cell's existing DNA to create a genetically modified, transgenic animal. The creation of transgenic animals also leads into the study of altering sperm cells with exogenous DNA.

Antigen presentation is a vital immune process that is essential for T cell immune response triggering. Because T cells recognize only fragmented antigens displayed on cell surfaces, antigen processing must occur before the antigen fragment can be recognized by a T-cell receptor. Specifically, the fragment, bound to the major histocompatibility complex (MHC), is transported to the surface of the antigen-presenting cell, a process known as presentation. If there has been an infection with viruses or bacteria, the cell antigen-presenting cell will present an endogenous or exogenous peptide fragment derived from the antigen by MHC molecules. There are two types of MHC molecules which differ in the behaviour of the antigens: MHC class I molecules (MHC-I) bind peptides from the cell cytosol, while peptides generated in the endocytic vesicles after internalisation are bound to MHC class II (MHC-II). Cellular membranes separate these two cellular environments - intracellular and extracellular. Each T cell can only recognize tens to hundreds of copies of a unique sequence of a single peptide among thousands of other peptides presented on the same cell, because an MHC molecule in one cell can bind to quite a large range of peptides. Predicting which antigens will be presented to the immune system by a certain MHC/HLA type is difficult, but the technology involved is improving.
Enclomifene (INNTooltip International Nonproprietary Name), or enclomiphene (USANTooltip United States Adopted Name), a nonsteroidal selective estrogen receptor modulator of the triphenylethylene group, acts by antagonizing the estrogen receptor (ER) in the pituitary gland, which reduces negative feedback by estrogen on the hypothalamic-pituitary-gonadal axis, thereby increasing gonadotropin secretion and hence gonadal production of testosterone. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Enclomifene is the (E)-stereoisomer of clomifene, while zuclomifene is the (Z)-stereoisomer. Whereas zuclomifene is more estrogenic, enclomifene is more antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the ER and reduces testosterone levels in men. As such, isomerically pure enclomifene is more favorable than clomifene as a progonadotropin for the treatment of male hypogonadism.
In a variety of contexts endogeneity is the property of being influenced within a system. It appears in specific contexts as such as economics, statistics, and social sciences. Specific examples are as follows:
A matrix metalloproteinase inhibitor inhibits matrix metalloproteinases. Because they inhibit cell migration, they have antiangiogenic effects. They are endogenous or exogenous.
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions from sub-disciplines and related fields, see Glossary of cell biology, Glossary of genetics, Glossary of evolutionary biology, Glossary of ecology, Glossary of environmental science and Glossary of scientific naming, or any of the organism-specific glossaries in Category:Glossaries of biology.

Estriol glucuronide (E3G), or oestriol glucuronide, also known as estriol monoglucuronide, as well as estriol 16α-β-D-glucosiduronic acid, is a natural, steroidal estrogen and the glucuronic acid conjugate of estriol. It occurs in high concentrations in the urine of pregnant women as a reversibly formed metabolite of estriol. Estriol glucuronide is a prodrug of estriol, and was the major component of Progynon and Emmenin, estrogenic products manufactured from the urine of pregnant women that were introduced in the 1920s and 1930s and were the first orally active estrogens. Emmenin was succeeded by Premarin, which is sourced from the urine of pregnant mares and was introduced in 1941. Premarin replaced Emmenin due to the fact that it was easier and less expensive to produce.

Cyclodiol is a synthetic estrogen which was studied in the 1990s and was never marketed. It is a derivative of estradiol with a bridge between the C14α and C17α positions. Cyclodiol has 100% of the relative binding affinity of estradiol for the human ERα and similar transactivational capacity as estradiol at the receptor. It has comparable potency to estradiol when administered by subcutaneous injection. The drug shows genotoxicity similarly to estradiol. Cyclodiol showed an absolute bioavailability of 33 ± 19% and an elimination half-life of 28.7 hours in pharmacokinetic studies in women.

Cyclotriol is a synthetic estrogen which was studied in the 1990s and was never marketed. It is a derivative of estriol with a bridge between the C14α and C17α positions. The drug has 40% of the relative binding affinity of estradiol for the human ERα. It showed an absolute bioavailability of 40% with high interindividual variability and an elimination half-life of 12.3 hours in pharmacokinetic studies in women.
References
- ↑ "Endogenous | Define Endogenous at Dictionary.com". Dictionary.reference.com. Retrieved 2011-07-11.