
The Controlled Substances Act (CSA) is the statute establishing federal U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated. It was passed by the 91st United States Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 and signed into law by President Richard Nixon. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs.

The Drug Enforcement Administration (DEA) is a United States federal law enforcement agency under the U.S. Department of Justice tasked with combating illicit drug trafficking and distribution within the U.S. It is the lead agency for domestic enforcement of the Controlled Substances Act, sharing concurrent jurisdiction with the Federal Bureau of Investigation, the U.S. Immigration and Customs Enforcement, and U.S. Customs and Border Protection. However, the DEA has sole responsibility for coordinating and pursuing U.S. drug investigations both domestically and internationally.

Orrin Grant Hatch was an American attorney and politician who served as a United States senator from Utah from 1977 to 2019. Hatch's 42-year Senate tenure made him the longest-serving Republican U.S. senator in history, overtaking Ted Stevens, until Chuck Grassley surpassed him in 2023.

Pentazocine, sold under the brand name Talwin among others, is a painkiller used to treat moderate to severe pain. It is believed to work by activating (agonizing) κ-opioid receptors (KOR) and μ-opioid receptors (MOR). As such it is called an opioid as it delivers its effects on pain by interacting with the opioid receptors. It shares many of the side effects of other opioids like constipation, nausea, itching, drowsiness and respiratory depression, but unlike most other opioids it fairly frequently causes hallucinations, nightmares and delusions. It is also, unlike most other opioids, subject to a ceiling effect, which is when at a certain dose no more pain relief is obtained by increasing the dose any further.

The Office of National Drug Control Policy (ONDCP) is a component of the Executive Office of the President of the United States.

Cardinal Health, Inc. is an American multinational health care services company, and the 14th highest revenue generating company in the United States. Headquartered in Dublin, Ohio, the company specializes in the distribution of pharmaceuticals and medical products, serving more than 100,000 locations. The company also manufactures medical and surgical product, including gloves, surgical apparel, and fluid management products. In addition, it operates one of the largest networks of radiopharmacies in the U.S. Cardinal Health provides medical products to over 75 percent of hospitals in the United States.

The war on drugs is the policy of a global campaign, led by the United States federal government, of drug prohibition, foreign assistance, and military intervention, with the aim of reducing the illegal drug trade in the US. The initiative includes a set of drug policies that are intended to discourage the production, distribution, and consumption of psychoactive drugs that the participating governments, through United Nations treaties, have made illegal.
Drug diversion is a medical and legal concept involving the transfer of any legally prescribed controlled substance from the individual for whom it was prescribed to another person for any illicit use. The definition varies slightly among different jurisdictions, but the transfer of a controlled substance alone usually does not constitute a diversion, since certain controlled substances that are prescribed to a child are intended to be administered by an adult, as directed by a medical professional. The term comes from the "diverting" of the drugs from their original licit medical purpose. In some jurisdictions, drug diversion programs are available to first time offenders of diversion drug laws, which "divert" offenders from the criminal justice system to a program of education and rehabilitation.

In the United States, the removal of cannabis from Schedule I of the Controlled Substances Act, the category reserved for drugs that have "no currently accepted medical use", is a proposed legal and administrative change in cannabis-related law at the federal level. After being proposed repeatedly since 1972, the U.S. Department of Justice initiated 2024 rulemaking to reschedule cannabis to Schedule III of the Controlled Substances Act. The majority of 2024 public comments supported descheduling, decriminalizing, or legalizing marijuana at the federal level.
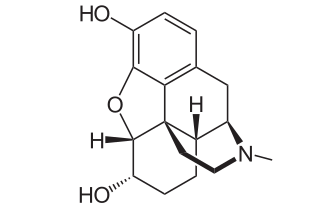
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Salvia divinorum, a psychoactive plant, is legal in most countries. Exceptions, countries where there is some form of control, include Australia, Belgium, Brazil, Canada, Denmark, Estonia, Finland, Germany, Iceland, Ireland, Italy, India, Japan, South Korea, Norway, Poland, United Kingdom, Ukraine, Spain, Sweden, Vietnam, Armenia and 33 states and territories of the United States.

In the United States, increased restrictions and labeling of cannabis as a poison began in many states from 1906 onward, and outright prohibitions began in the 1920s. By the mid-1930s cannabis was regulated as a drug in every state, including 35 states that adopted the Uniform State Narcotic Drug Act. The first national regulation was the Marihuana Tax Act of 1937.
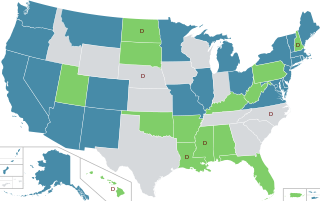
In the United States, the use of cannabis for medical purposes is legal in 38 states, four out of five permanently inhabited U.S. territories, and the District of Columbia, as of March 2023. Ten other states have more restrictive laws limiting THC content, for the purpose of allowing access to products that are rich in cannabidiol (CBD), a non-psychoactive component of cannabis. There is significant variation in medical cannabis laws from state to state, including how it is produced and distributed, how it can be consumed, and what medical conditions it can be used for.

Thomas Anthony Marino is an American politician and attorney, who served as a United States Representative from Pennsylvania from 2011 to 2019. He represented the 10th congressional district from January 3, 2011 to January 3, 2019, and the 12th district from January 3 to January 23, 2019, when he resigned to work in the private sector. A member of the Republican Party, Marino was the United States Attorney for the United States District Court for the Middle District of Pennsylvania in his early career.

The 21st Century Cures Act is a United States law enacted by the 114th United States Congress in December 2016 and then signed into law on December 13, 2016. It authorized $6.3 billion in funding, mostly for the National Institutes of Health. The act was supported especially by large pharmaceutical manufacturers and was opposed especially by some consumer organizations.

There is an ongoing opioid epidemic in the United States, originating out of both medical prescriptions and illegal sources. It has been called "one of the most devastating public health catastrophes of our time". The opioid epidemic unfolded in three waves. The first wave of the epidemic in the United States began in the late 1990s, according to the Centers for Disease Control and Prevention (CDC), when opioids were increasingly prescribed for pain management, resulting in a rise in overall opioid use throughout subsequent years. The second wave was from an expansion in the heroin market to supply already addicted people. The third wave starting in 2013 was marked by a steep 1,040% increase in the synthetic opioid-involved death rate as synthetic opioids flooded the US market.

Drug disposal is the discarding of drugs. Individuals commonly dispose of unused drugs that remain after the end of medical treatment. Health care organizations dispose of drugs on a larger scale for a range of reasons, including having leftover drugs after treating patients and discarding of expired drugs. Failure to properly dispose of drugs creates opportunities for others to take them inappropriately. Inappropriate disposal of drugs can also cause drug pollution.
SUPPORT for Patients and Communities Act, also known as Substance Use–Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, is a United States federal law, enacted during the 115th United States Congress, to make medical treatment for opioid addiction more widely available while also cracking down on illicit drugs. This piece of legislation is part of the ongoing conflict to stop and prevent the opioid epidemic in the United States. President Trump signed the bill on October 24, 2018.

The opioid epidemic, also referred to as the opioid crisis, is the rapid increase in the overuse, misuse/abuse, and overdose deaths attributed either in part or in whole to the class of drugs called opiates/opioids since the 1990s. It includes the significant medical, social, psychological, demographic and economic consequences of the medical, non-medical, and recreational abuse of these medications.

The Crime of the Century is an American two-part documentary film directed, produced, and written by Alex Gibney. The film follows the opioid epidemic in the United States, and the political operatives, government regulations and corporations that enable the abuse of opioids, particularly the Sackler family and Purdue Pharma.
















