
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.

Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.
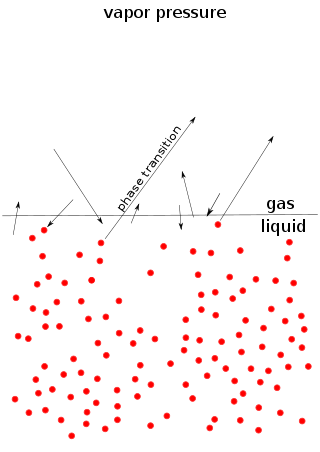
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.

Boiling or ebullition is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.
Vaporization of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, where as boiling is a bulk phenomenon.

The van der Waals equation, named for its originator, the Dutch physicist Johannes Diderik van der Waals, is an equation of state that extends the ideal gas law to include the non-zero size of gas molecules and the interactions between them. As a result the equation is able to model the phase change, liquid vapor. It also produces simple analytic expressions for the properties of real substances that shed light on their behavior. One way to write this equation is:
In thermodynamics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling. This is a so-called metastable state or metastate, where boiling might occur at any time, induced by external or internal effects. Superheating is achieved by heating a homogeneous substance in a clean container, free of nucleation sites, while taking care not to disturb the liquid.

A boiling liquid expanding vapor explosion is an explosion caused by the rupture of a vessel containing a pressurized liquid that is or has reached a temperature sufficiently higher than its boiling point. Because the boiling point of a liquid rises with pressure, the contents of the pressurized vessel can remain a liquid as long as the vessel is intact. If the vessel's integrity is compromised, the loss of pressure drops the boiling point, which can cause the liquid to convert to gas expanding rapidly. BLEVEs are manifestations of explosive boiling.
In physics and chemistry, flash freezing is the process whereby objects are rapidly frozen. This is done by subjecting them to cryogenic temperatures, or it can be done through direct contact with liquid nitrogen at −196 °C (−320.8 °F). It is commonly used in the food industry.

Crystallization is the process by which solids form, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, cooling rate, and in the case of liquid crystals, time of fluid evaporation.

A steam explosion is an explosion caused by violent boiling or flashing of water or ice into steam, occurring when water or ice is either superheated, rapidly heated by fine hot debris produced within it, or heated by the interaction of molten metals. Steam explosions are instances of explosive boiling. Pressure vessels, such as pressurized water (nuclear) reactors, that operate above atmospheric pressure can also provide the conditions for a steam explosion. The water changes from a solid or liquid to a gas with extreme speed, increasing dramatically in volume. A steam explosion sprays steam and boiling-hot water and the hot medium that heated it in all directions, creating a danger of scalding and burning.

In thermodynamics, a critical point is the end point of a phase equilibrium curve. One example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a critical temperatureTc and a critical pressurepc, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition in the absence of an external magnetic field.

In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled below 0 °C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0 °C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay.
The Wegener–Bergeron–Findeisen process, is a process of ice crystal growth that occurs in mixed phase clouds in regions where the ambient vapor pressure falls between the saturation vapor pressure over water and the lower saturation vapor pressure over ice. This is a subsaturated environment for liquid water but a supersaturated environment for ice resulting in rapid evaporation of liquid water and rapid ice crystal growth through vapor deposition. If the number density of ice is small compared to liquid water, the ice crystals can grow large enough to fall out of the cloud, melting into rain drops if lower level temperatures are warm enough.
The Kelvin equation describes the change in vapour pressure due to a curved liquid–vapor interface, such as the surface of a droplet. The vapor pressure at a convex curved surface is higher than that at a flat surface. The Kelvin equation is dependent upon thermodynamic principles and does not allude to special properties of materials. It is also used for determination of pore size distribution of a porous medium using adsorption porosimetry. The equation is named in honor of William Thomson, also known as Lord Kelvin.
In fluid thermodynamics, nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux. For water, as shown in the graph below, nucleate boiling occurs when the surface temperature is higher than the saturation temperature by between 10 and 30 °C. The critical heat flux is the peak on the curve between nucleate boiling and transition boiling. The heat transfer from surface to liquid is greater than that in film boiling.

In thermodynamic equilibrium, a necessary condition for stability is that pressure, , does not increase with molar volume, ; this is expressed mathematically as , where is the temperature.

Steam is water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Steam that is saturated or superheated is invisible; however, wet steam, a visible mist or aerosol of water droplets, is often referred to as "steam".

In thermodynamics, the limit of local stability with respect to small fluctuations is clearly defined by the condition that the second derivative of Gibbs free energy is zero.

In thermodynamics, the binodal, also known as the coexistence curve or binodal curve, denotes the condition at which two distinct phases may coexist. Equivalently, it is the boundary between the set of conditions in which it is thermodynamically favorable for the system to be fully mixed and the set of conditions in which it is thermodynamically favorable for it to phase separate. In general, the binodal is defined by the condition at which the chemical potential of all solution components is equal in each phase. The extremum of a binodal curve in temperature coincides with the one of the spinodal curve and is known as a critical point.













