Related Research Articles

Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.

Natural gas is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane (95%) in addition to various smaller amounts of other higher alkanes. Traces of carbon dioxide, nitrogen, hydrogen sulfide, and helium are also usually present. Methane is colorless and odorless, and the second largest greenhouse gas contributor to global climate change after carbon dioxide. Because natural gas is odorless, odorizers such as mercaptan are commonly added to it for safety so that leaks can be readily detected.

Propane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases. The others include propylene, butane, butylene, butadiene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal.

Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.

Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition.

Boiling or ebullition is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.

Liquid hydrogen (H2(l)) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.

Latent heat is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation.

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
Vaporization of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, where as boiling is a bulk phenomenon.

Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. The verb form of sublimation is sublime, or less preferably, sublimate. Sublimate also refers to the product obtained by sublimation. The point at which sublimation occurs rapidly is called critical sublimation point, or simply sublimation point. Notable examples include sublimation of dry ice at room temperature and atmospheric pressure, and that of solid iodine with heating.
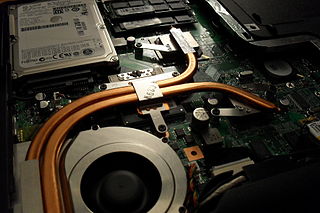
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

A boiling liquid expanding vapor explosion is an explosion caused by the rupture of a vessel containing a pressurized liquid that is or has reached a temperature sufficiently higher than its boiling point at atmospheric pressure. Because the boiling point of a liquid rises with pressure, the contents of the pressurized vessel can remain a liquid as long as the vessel is intact. If the vessel's integrity is compromised, the loss of pressure drops the boiling point, which can cause the liquid to convert to gas expanding rapidly. BLEVEs are manifestations of explosive boiling.

Liquefied natural gas (LNG) is natural gas (predominantly methane, CH4, with some mixture of ethane, C2H6) that has been cooled down to liquid form for ease and safety of non-pressurized storage or transport. It takes up about 1/600th the volume of natural gas in the gaseous state at standard conditions for temperature and pressure.
The heating value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specified amount of it.

A steam explosion is an explosion caused by violent boiling or flashing of water or ice into steam, occurring when water or ice is either superheated, rapidly heated by fine hot debris produced within it, or heated by the interaction of molten metals. Steam explosions are instances of explosive boiling. Pressure vessels, such as pressurized water (nuclear) reactors, that operate above atmospheric pressure can also provide the conditions for a steam explosion. The water changes from a solid or liquid to a gas with extreme speed, increasing dramatically in volume. A steam explosion sprays steam and boiling-hot water and the hot medium that heated it in all directions, creating a danger of scalding and burning.
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
A liquefied natural gas (LNG) spill can happen during an accident or an intentional act. LNG is normally stored and transported in liquid form at a temperature of approximately −162 °C (−260 °F). If this cooled liquid is released from a storage facility, pipeline, or LNG transport ship, then it begins to warm. As LNG warms above its storage temperature, the liquid begins to vaporize. The resulting gas produced by this warming is typically methane, which is the major component of natural gas and one of the most potent and hazardous greenhouse gases.

A liquefied natural gas terminal is a facility for managing the import and/or export of liquefied natural gas (LNG). It comprises equipment for loading and unloading of LNG cargo to/from ocean-going tankers, for transfer across the site, liquefaction, re-gasification, processing, storage, pumping, compression, and metering of LNG. LNG as a liquid is the most efficient way to transport natural gas over long distances, usually by sea.
References
- ↑ Melhem, G.A.; Saraf, F.; Ozog, H. (2006). LNG Properties and Hazards (PDF) (Report). ioMosaic. Archived from the original (PDF) on 28 August 2013.
- ↑ "Glossary of LNG-Related Terms & Definitions". California Energy Commission . 5 January 2004. Archived from the original on 22 August 2004.