GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved G domain common to many GTPases.
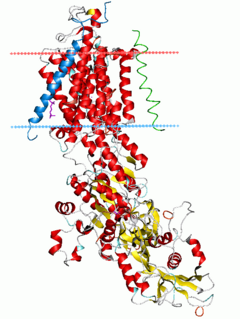
Na⁺/K⁺-ATPase is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.

Cyclic nucleotide–gated ion channels or CNG channels are ion channels that function in response to the binding of cyclic nucleotides. CNG channels are nonselective cation channels that are found in the membranes of various tissue and cell types, and are significant in sensory transduction as well as cellular development. Their function can be the result of a combination of the binding of cyclic nucleotides and either a depolarization or a hyperpolarization event. Initially discovered in the cells that make up the retina of the eye, CNG channels have been found in many different cell types across both the animal and the plant kingdoms. CNG channels have a very complex structure with various subunits and domains that play a critical role in their function. CNG channels are significant in the function of various sensory pathways including vision and olfaction, as well as in other key cellular functions such as hormone release and chemotaxis. CNG channels have also been found to exist in prokaryotes, including many spirochaeta, though their precise role in bacterial physiology remains unknown.
Phospholipase D (PLD) is an enzyme of the phospholipase superfamily. Phospholipases occur widely, and can be found in a wide range of organisms, including bacteria, yeast, plants, animals, and viruses. Phospholipase D's principal substrate is phosphatidylcholine, which it hydrolyzes to produce the signal molecule phosphatidic acid (PA), and soluble choline. Plants contain numerous genes that encode various PLD isoenzymes, with molecular weights ranging from 90 to 125 kDa. Mammalian cells encode two isoforms of phospholipase D: PLD1 and PLD2. Phospholipase D is an important player in many physiological processes, including membrane trafficking, cytoskeletal reorganization, receptor-mediated endocytosis, exocytosis, and cell migration. Through these processes, it has been further implicated in the pathophysiology of multiple diseases: in particular the progression of Parkinson's and Alzheimer's, as well as various cancers. PLD may also help set the threshold for sensitivity to anesthetia and mechanical force.

Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the plasma membranes and to anchor specific ion channels, ion exchangers and ion transporters in the plasma membrane. The name is derived from the Greek word for "fused".

The epithelial sodium channel (short: ENaC, also: amiloride-sensitive sodium channel) is a membrane-bound ion channel that is selectively permeable to the ions of sodium (Na+) and that is assembled as a heterotrimer composed of three homologous subunits α or δ, β, and γ, These subunits are encoded by four genes: SCNN1A, SCNN1B, SCNN1G, and SCNN1D. It is involved primarily in the reabsorption of sodium ions at the collecting ducts of the kidney's nephrons.
In molecular biology, the electroneutral cation-Cl family of proteins are a family of solute carrier proteins. This family includes the products of the Human genes: SLC12A1, SLC12A1, SLC12A2, SLC12A3, SLC12A4, SLC12A5, SLC12A6, SLC12A7, SLC12A8 and SLC12A9.
FXYD6, or FXYD domain-containing ion transport regulator 6, is a gene which is located at the 11q23.3. The FXYD6 protein contains 95 amino acids, and can be found in all human tissues except blood.

Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
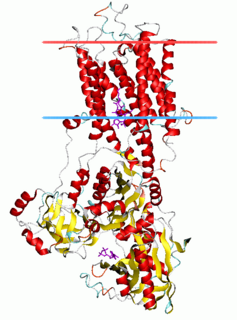
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) Superfamily (TC# 3.A.3) which, as of early 2016, includes 20 different protein families.

Sodium/potassium-transporting ATPase subunit alpha-1 is an enzyme that in humans is encoded by the ATP1A1 gene.

Sodium/potassium-transporting ATPase subunit beta-1 is an enzyme that in humans is encoded by the ATP1B1 gene.

Solute carrier family 23 member 2 is a protein that in humans is encoded by the SLC23A2 gene.

Sodium/potassium-transporting ATPase subunit alpha-3 is an enzyme that in humans is encoded by the ATP1A3 gene.

Sodium/potassium-transporting ATPase subunit alpha-4 is an enzyme that in humans is encoded by the ATP1A4 gene.
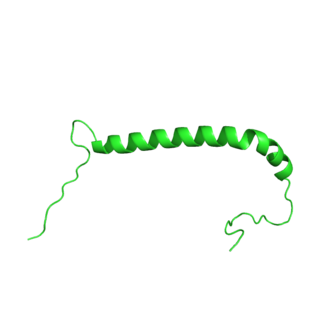
Sodium/potassium-transporting ATPase gamma chain is a protein that in humans is encoded by the FXYD2 gene.

FXYD domain-containing ion transport regulator 5 also named dysadherin (human) or RIC (mouse) is a protein that in humans is encoded by the FXYD5 gene.

Phospholemman (PLM) is a protein that in humans is encoded by the FXYD1 gene.

Sodium/potassium-transporting ATPase subunit beta-3 is an enzyme that in humans is encoded by the ATP1B3 gene. ATP1B3 has also been designated as CD298.

FXYD domain-containing ion transport regulator 3 is a protein that in humans is encoded by the FXYD3 gene.













