In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.

Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver and are a major component of human skin oils.
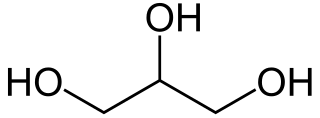
Glycerol, also called glycerine or glycerin, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of enzymes, particularly lipases.
Saponification is a process of cleaving esters into carboxylate salts and alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol:
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This process renders a product (chemistry) and by-products.

Saponification value or saponification number represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value are more suitable for soap making.

Ethyl oleate is a fatty acid ester formed by the condensation of oleic acid and ethanol. It is a colorless oil although degraded samples can appear yellow.
In chemistry, solvolysis is a type of nucleophilic substitution (SN1/SN2) or elimination where the nucleophile is a solvent molecule. Characteristic of SN1 reactions, solvolysis of a chiral reactant affords the racemate. Sometimes however, the stereochemical course is complicated by intimate ion pairs, whereby the leaving anion remains close to the carbocation, effectively shielding it from an attack by the nucleophile. Particularly fast reactions can occur by neighbour group participation, with nonclassical ions as intermediates or transition states.
In chemistry, acid value is a number used to quantify the acidity of a given chemical substance. It is the quantity of base, expressed as milligrams of KOH required to neutralize the acidic constituents in 1 gram of a sample. The acid value measures the acidity of water-insoluble substances like oils, fats, waxes and resins, which do not have a pH value.

Monoglycerides are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol.
EN 14214 is a standard published by the European Committee for Standardization that describes the requirements and test methods for FAME - the most common type of biodiesel.
Oleochemistry is the study of vegetable oils and animal oils and fats, and oleochemicals derived from these fats and oils. The resulting product can be called oleochemicals (from Latin: oleum "olive oil"). The major product of this industry is soap, approximately 8.9×106 tons of which were produced in 1990. Other major oleochemicals include fatty acids, fatty acid methyl esters, fatty alcohols and fatty amines. Glycerol is a side product of all of these processes. Intermediate chemical substances produced from these basic oleochemical substances include alcohol ethoxylates, alcohol sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols (MAG), diacylglycerols (DAG), structured triacylglycerols (TAG), sugar esters, and other oleochemical products.

In biochemistry, lipase refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms.

Ethyl glucuronide (EtG) is a metabolite of ethanol which is formed in the body by glucuronidation following exposure to ethanol, usually from drinking alcoholic beverages. It is used as a biomarker to test for ethanol use and to monitor alcohol abstinence in situations where drinking is prohibited, such as by the military, in alcohol treatment programs, in professional monitoring programs, in schools, liver transplant clinics, or in recovering alcoholic patients. In addition to its use to monitor abstinence and detect drinking, EtG also has potential for monitoring the amount of alcohol use over time because it can be detected in hair and nails, though the effectiveness of this has not yet been proven.

Phosphatidylethanols (PEth) are a group of phospholipids formed only in the presence of ethanol via the action of phospholipase D (PLD). It accumulates in blood and is removed slowly, making it a useful biomarker for alcohol consumption. PEth is also thought to contribute to the symptoms of alcohol intoxication.
Single cell oil, also known as Microbial oil consists of the intracellular storage lipids, triacyglycerols. It is similar to vegetable oil, another biologically produced oil. They are produced by oleaginous microorganisms, which is the term for those bacteria, molds, algae and yeast, which can accumulate 20% to 80% lipids of their biomass. The accumulation of lipids take place by the end of logarithmic phase and continues during station phase until carbon source begins to reduce with nutrition limitation.

A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009.

N-Acetyltaurine (NAcT) is an endogenous metabolite. Biochemically, N-acetyltaurine is formed as a result of an acetylation of taurine. The main substrate for this reaction is acetate. An increase of endogenous N-acetyltaurine concentrations was observed after the consumption of alcohol and after extended physical activity (ketoacidosis).