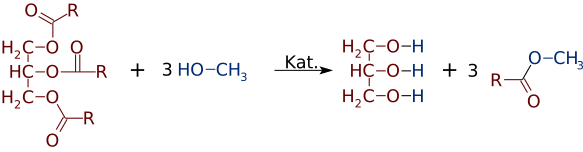In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Biofuel is a fuel that is produced over a short time span from biomass, rather than by the very slow natural processes involved in the formation of fossil fuels such as oil. Biofuel can be produced from plants or from agricultural, domestic or industrial biowaste. Biofuels are mostly used for transportation, but can also be used for heating and electricity. Biofuels are regarded as a renewable energy source. The use of biofuel has been subject to criticism regarding the "food vs fuel" debate, varied assessments of their sustainability, and ongoing deforestation and biodiversity loss as a result of biofuel production.

Biodiesel is a renewable biofuel, a form of diesel fuel, derived from biological sources like vegetable oils, animal fats, or recycled greases, and consisting of long-chain fatty acid esters. It is typically made from fats.
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of enzymes, particularly lipases.
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This process renders a product (chemistry) and by-products.
In chemistry, solvolysis is a type of nucleophilic substitution (SN1/SN2) or elimination where the nucleophile is a solvent molecule. Characteristic of SN1 reactions, solvolysis of a chiral reactant affords the racemate. Sometimes however, the stereochemical course is complicated by intimate ion pairs, whereby the leaving anion remains close to the carbocation, effectively shielding it from an attack by the nucleophile. Particularly fast reactions can occur by neighbour group participation, with nonclassical ions as intermediates or transition states.
In chemistry, acid value is a number used to quantify the acidity of a given chemical substance. It is the quantity of base, expressed as milligrams of KOH required to neutralize the acidic constituents in 1 gram of a sample. The acid value measures the acidity of water-insoluble substances like oils, fats, waxes and resins, which do not have a pH value.
The word metagenics uses the prefix meta and the suffix gen. Literally, it means "the creation of something which creates". In the context of biotechnology, metagenics is the practice of engineering organisms to create a specific enzyme, protein, or other biochemicals from simpler starting materials. The genetic engineering of E. coli with the specific task of producing human insulin from starting amino acids is an example. E. coli has also been engineered to digest plant biomass and use it to produce hydrocarbons in order to synthesize biofuels. The applications of metagenics on E. coli also include higher alcohols, fatty-acid based chemicals and terpenes.
EN 14214 is a standard published by the European Committee for Standardization that describes the requirements and test methods for FAME - the most common type of biodiesel.
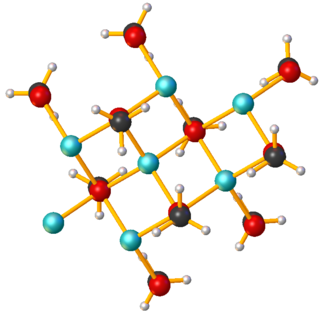
Sodium methoxide is the simplest sodium alkoxide. With the formula CH3ONa, it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.

Jatropha curcas is a species of flowering plant in the spurge family, Euphorbiaceae, that is native to the American tropics, most likely Mexico and Central America. It is originally native to the tropical areas of the Americas from Mexico to Argentina, and has been spread throughout the world in tropical and subtropical regions around the world, becoming naturalized or invasive in many areas. The specific epithet, "curcas", was first used by Portuguese doc Garcia de Orta more than 400 years ago. Common names in English include physic nut, Barbados nut, poison nut, bubble bush or purging nut. In parts of Africa and areas in Asia such as India it is often known as "castor oil plant" or "hedge castor oil plant", but it is not the same as the usual castor oil plant, Ricinus communis.

Choricystis is a genus of green algae in the class Trebouxiophyceae, considered a characteristic picophytoplankton in freshwater ecosystems. Choricystis, especially the type species Choricystis minor, has been proposed as an effective source of fatty acids for biofuels. Choricystis algacultures have been shown to survive on wastewater. In particular, Choricystis has been proposed as a biological water treatment system for industrial waste produced by the processing of dairy goods.

Fatty acid esters (FAEs) are a type of ester that result from the combination of a fatty acid with an alcohol. When the alcohol component is glycerol, the fatty acid esters produced can be monoglycerides, diglycerides, or triglycerides. Dietary fats are chemically triglycerides.
Oleochemistry is the study of vegetable oils and animal oils and fats, and oleochemicals derived from these fats and oils. The resulting product can be called oleochemicals (from Latin: oleum "olive oil"). The major product of this industry is soap, approximately 8.9×106 tons of which were produced in 1990. Other major oleochemicals include fatty acids, fatty acid methyl esters, fatty alcohols and fatty amines. Glycerol is a side product of all of these processes. Intermediate chemical substances produced from these basic oleochemical substances include alcohol ethoxylates, alcohol sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols (MAG), diacylglycerols (DAG), structured triacylglycerols (TAG), sugar esters, and other oleochemical products.

Chicken fat is fat obtained from chicken rendering and processing. Of the many animal-sourced substances, chicken fat is noted for being high in linoleic acid, an omega-6 fatty acid. Linoleic acid levels are between 17.9% and 22.8%. It is a common flavoring, additive or main component of chicken soup. It is often used in pet foods, and has been used in the production of biodiesel. One method of converting chicken fat into biodiesel is through a process called supercritical methanol treatment.
LS-9 Inc was a venture-funded company focused on producing diesel fuel from transgenic organisms. It launched in 2005, took in $81 million in investment, and in 2013 was sold to Renewable Energy Group for $40 million in cash and stock, and an additional $21.5 million if technology and production milestones were met.

Phospholipid-derived fatty acids (PLFAs) are widely used in microbial ecology as chemotaxonomic markers of bacteria and other organisms. Phospholipids are the primary lipids composing cellular membranes. Phospholipids can be saponified, which releases the fatty acids contained in their diglyceride tail. Once the phospholipids of an unknown sample are saponified, the composition of the resulting PLFA can be compared to the PLFA of known organisms to determine the identity of the sample organism. PLFA analysis may be combined with other techniques, such as stable isotope probing to determine which microbes are metabolically active in a sample. PLFA analysis was pioneered by D.C. White at the University of Tennessee, in the early to mid 1980s.
Potassium methoxide is the alkoxide of methanol with the counterion potassium and is used as a strong base and as a catalyst for transesterification, in particular for the production of biodiesel.
Single cell oil, also known as Microbial oil consists of the intracellular storage lipids, triacyglycerols. It is similar to vegetable oil, another biologically produced oil. They are produced by oleaginous microorganisms, which is the term for those bacteria, molds, algae and yeast, which can accumulate 20% to 80% lipids of their biomass. The accumulation of lipids take place by the end of logarithmic phase and continues during station phase until carbon source begins to reduce with nutrition limitation.
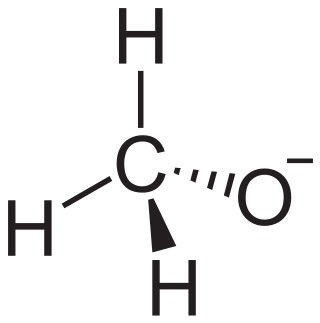
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.