Related Research Articles
In electromagnetism, the magnetic susceptibility is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization M to the applied magnetizing field intensity H. This allows a simple classification, into two categories, of most materials' responses to an applied magnetic field: an alignment with the magnetic field, χ > 0, called paramagnetism, or an alignment against the field, χ < 0, called diamagnetism.

Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic part of many spin interactions are present in solid-state NMR, unlike in solution-state NMR where rapid tumbling motion averages out many of the spin interactions. As a result, solid-state NMR spectra are characterised by larger linewidths than in solution state NMR, which can be utilized to give quantitative information on the molecular structure, conformation and dynamics of the material. Solid-state NMR is often combined with magic angle spinning to remove anisotropic interactions and improve the resolution as well as the sensitivity of the technique.

Magnetic force microscopy (MFM) is a variety of atomic force microscopy, in which a sharp magnetized tip scans a magnetic sample; the tip-sample magnetic interactions are detected and used to reconstruct the magnetic structure of the sample surface. Many kinds of magnetic interactions are measured by MFM, including magnetic dipole–dipole interaction. MFM scanning often uses non-contact AFM (NC-AFM) mode.
Nuclear magnetic resonance spectroscopy of proteins is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and their complexes. The field was pioneered by Richard R. Ernst and Kurt Wüthrich at the ETH, and by Ad Bax, Marius Clore, Angela Gronenborn at the NIH, and Gerhard Wagner at Harvard University, among others. Structure determination by NMR spectroscopy usually consists of several phases, each using a separate set of highly specialized techniques. The sample is prepared, measurements are made, interpretive approaches are applied, and a structure is calculated and validated.
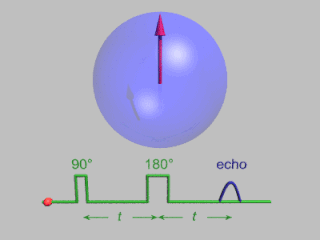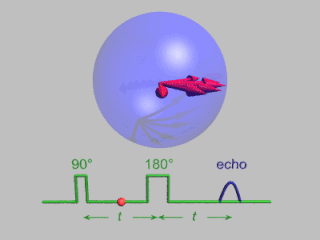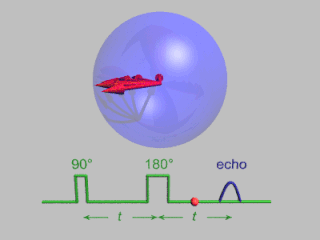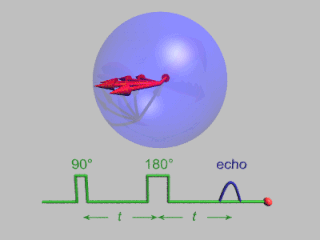
In magnetic resonance, a spin echo or Hahn echo is the refocusing of spin magnetisation by a pulse of resonant electromagnetic radiation. Modern nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) make use of this effect.
Spin-polarized scanning tunneling microscopy (SP-STM) is a type of scanning tunneling microscope (STM) that can provide detailed information of magnetic phenomena on the single-atom scale additional to the atomic topography gained with STM. SP-STM opened a novel approach to static and dynamic magnetic processes as precise investigations of domain walls in ferromagnetic and antiferromagnetic systems, as well as thermal and current-induced switching of nanomagnetic particles.
Fast low angle shot magnetic resonance imaging is a particular sequence of magnetic resonance imaging. It is a gradient echo sequence which combines a low-flip angle radio-frequency excitation of the nuclear magnetic resonance signal with a short repetition time. It is the generic form of steady-state free precession imaging.

In physics, the spin–spin relaxation is the mechanism by which Mxy, the transverse component of the magnetization vector, exponentially decays towards its equilibrium value in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI). It is characterized by the spin–spin relaxation time, known as T2, a time constant characterizing the signal decay. It is named in contrast to T1, the spin–lattice relaxation time. It is the time it takes for the magnetic resonance signal to irreversibly decay to 37% (1/e) of its initial value after its generation by tipping the longitudinal magnetization towards the magnetic transverse plane. Hence the relation
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.

In magnetic resonance imaging (MRI), k-space is the 2D or 3D Fourier transform of the image measured. It was introduced in 1979 by Likes and in 1983 by Ljunggren and Twieg.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
In nuclear magnetic resonance spectroscopy and magnetic resonance imaging, the Ernst angle is the flip angle for excitation of a particular spin that gives the maximal signal intensity in the least amount of time when signal averaging over many transients. In other words, the highest signal-to-noise ratio can be achieved in a given amount of time. This relationship was described by Richard R. Ernst, winner of the 1991 Nobel Prize in Chemistry.

The physics of magnetic resonance imaging (MRI) concerns fundamental physical considerations of MRI techniques and technological aspects of MRI devices. MRI is a medical imaging technique mostly used in radiology and nuclear medicine in order to investigate the anatomy and physiology of the body, and to detect pathologies including tumors, inflammation, neurological conditions such as stroke, disorders of muscles and joints, and abnormalities in the heart and blood vessels among others. Contrast agents may be injected intravenously or into a joint to enhance the image and facilitate diagnosis. Unlike CT and X-ray, MRI uses no ionizing radiation and is, therefore, a safe procedure suitable for diagnosis in children and repeated runs. Patients with specific non-ferromagnetic metal implants, cochlear implants, and cardiac pacemakers nowadays may also have an MRI in spite of effects of the strong magnetic fields. This does not apply on older devices, and details for medical professionals are provided by the device's manufacturer.

Pulsed electron paramagnetic resonance (EPR) is an electron paramagnetic resonance technique that involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short oscillating field, usually a microwave pulse. One can then measure the emitted microwave signal which is created by the sample magnetization. Fourier transformation of the microwave signal yields an EPR spectrum in the frequency domain. With a vast variety of pulse sequences it is possible to gain extensive knowledge on structural and dynamical properties of paramagnetic compounds. Pulsed EPR techniques such as electron spin echo envelope modulation (ESEEM) or pulsed electron nuclear double resonance (ENDOR) can reveal the interactions of the electron spin with its surrounding nuclear spins.
The Shinnar–Le Roux (SLR) algorithm is a mathematical tool for generating frequency-selective radio frequency (RF) pulses in magnetic resonance imaging (MRI). Frequency selective pulses are used in MRI to isolate a slice through the subject for excitation, inversion and saturation.
Hyperpolarized carbon-13 MRI is a functional medical imaging technique for probing perfusion and metabolism using injected substrates.
Adiabatic radio frequency (RF) pulses are used in magnetic resonance imaging (MRI) to achieve excitation that is insensitive to spatial inhomogeneities in the excitation field or off-resonances in the sampled object.

An MRI sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
Gradient echo is a magnetic resonance imaging (MRI) sequence that has wide variety of applications, from magnetic resonance angiography to perfusion MRI and diffusion MRI. Rapid imaging acquisition allows it to be applied to 2D and 3D MRI imaging. Gradient echo uses magnetic gradients to generate a signal, instead of using 180 degrees radiofrequency pulse like spin echo; thus leading to faster image acquisition time.
An MRI artifact is a visual artifact in magnetic resonance imaging (MRI). It is a feature appearing in an image that is not present in the original object. Many different artifacts can occur during MRI, some affecting the diagnostic quality, while others may be confused with pathology. Artifacts can be classified as patient-related, signal processing-dependent and hardware (machine)-related.
References
- ↑ Weishaupt, Dominik; Koechli, Victor D.; Froehlich, J. M.; Nanz, D.; Marincek, Borut; Pruessmann, K. P. (February 2008). How Does MRI Work? An Introduction to the Physics and Function of Magnetic Resonance Imaging. Springer Berlin Heidelberg. p. 151. ISBN 9783540378457 . Retrieved 11 April 2023.
- ↑ Runge, Val M.; Heverhagen, Johannes T. (21 May 2022). The Physics of Clinical MR Taught Through Images. Springer International Publishing. p. 87. ISBN 9783030854133 . Retrieved 11 April 2023.