Related Research Articles

Xanthophylls are yellow pigments that occur widely in nature and form one of two major divisions of the carotenoid group; the other division is formed by the carotenes. The name is from Greek xanthos and phyllon, due to their formation of the yellow band seen in early chromatography of leaf pigments.
Phycobilisomes are light harvesting antennae of photosystem II in cyanobacteria, red algae and glaucophytes.

Glutamine synthetase (GS) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine:
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress.

Photoinhibition is light-induced reduction in the photosynthetic capacity of a plant, alga, or cyanobacterium. Photosystem II (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the photosynthetic reaction center of PSII. Photoinhibition is also used in a wider sense, as dynamic photoinhibition, to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light.
DnaE, the gene product of dnaE, is the catalytic α subunit of DNA polymerase III, acting as a DNA polymerase. This enzyme is only found in prokaryotes.
In enzymology, a glucosylglycerol 3-phosphatase (EC 3.1.3.69) is an enzyme that catalyzes the chemical reaction
Synechocystis sp. PCC6803 is a strain of unicellular, freshwater cyanobacteria. Synechocystis sp. PCC6803 is capable of both phototrophic growth by oxygenic photosynthesis during light periods and heterotrophic growth by glycolysis and oxidative phosphorylation during dark periods. Gene expression is regulated by a circadian clock and the organism can effectively anticipate transitions between the light and dark phases.
Non-photochemical quenching (NPQ) is a mechanism employed by plants and algae to protect themselves from the adverse effects of high light intensity. It involves the quenching of singlet excited state chlorophylls (Chl) via enhanced internal conversion to the ground state, thus harmlessly dissipating excess excitation energy as heat through molecular vibrations. NPQ occurs in almost all photosynthetic eukaryotes, and helps to regulate and protect photosynthesis in environments where light energy absorption exceeds the capacity for light utilization in photosynthesis.
Cyanobacteriochromes are phytochrome-related photoreceptor proteins found only in the cyanobacteria.

The psaA RNA motif describes a class of RNAs with a common secondary structure. psaA RNAs are exclusively found in locations that presumably correspond to the 5' untranslated regions of operons formed of psaA and psaB genes. For this reason, it was hypothesized that psaA RNAs function as cis-regulatory elements of these genes. The psaAB genes encode proteins that form subunits in the photosystem I structure used for photosynthesis. psaA RNAs have been detected only in cyanobacteria, which is consistent with their association with photosynthesis.
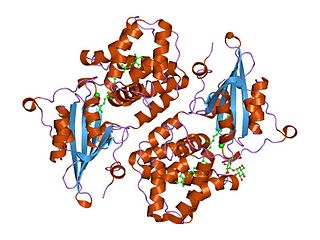
In molecular biology the orange carotenoid N-terminal domain is a protein domain found predominantly at the N-terminus of the Orange carotenoid protein (OCP), and is involved in non-covalent binding of a carotenoid chromophore. It is unique for being present in soluble proteins, whereas the vast majority of domains capable of binding carotenoids are intrinsic membrane proteins. Thus far, it has exclusively been found in cyanobacteria, among which it is widespread. The domain also exists on its own, in uncharacterized cyanobacterial proteins referred to as "Red Carotenoid Protein" (RCP). The domain adopts an alpha-helical structure consisting of two four-helix bundles.
Plastoglobulins is a family of proteins prominent found in lipid globules in plastids of flowering plants. It shows sequence similarities to the PAP/fibrillin family. PGL and similar proteins can be found in most algae, cyanobacteria and plants, but no other life forms; it suggests a role for PGL in oxygenic photosynthesis.
In molecular biology, Cyanobacterial non-coding RNAs are non-coding RNAs which have been identified in species of cyanobacteria. Large scale screens have identified 21 Yfr in the marine cyanobacterium Prochlorococcus and related species such as Synechococcus. These include the Yfr1 and Yfr2 RNAs. In Prochlorococcus and Synechocystis, non-coding RNAs have been shown to regulate gene expression. NsiR4, widely conserved throughout the cyanobacterial phylum, has been shown to be involved in nitrogen assimilation control in Synechocystis sp. PCC 6803 and in the filamentous, nitrogen-fixing Anabaena sp. PCC 7120.
kaiA is a gene in the "kaiABC" gene cluster that plays a crucial role in the regulation of bacterial circadian rhythms, such as in the cyanobacterium Synechococcuselongatus. For these bacteria, regulation of kaiA expression is critical for circadian rhythm, which determines the twenty-four-hour biological rhythm. In addition, KaiA functions with a negative feedback loop in relation with kaiB and KaiC. The kaiA gene makes KaiA protein that enhances phosphorylation of KaiC while KaiB inhibits activity of KaiA.
Iron-starvation-induced protein A, also known as isiA, is a photosynthesis-related chlorophyll-containing protein found in cyanobacteria. It belongs to the chlorophyll-a/b-binding family of proteins, and has been shown to have a photoprotection role in preventing oxidative damage via energy dissipation. It was originally identified under Fe starvation, and thus received the name iron-starvation-induced protein A. However, the protein has more recently been found to respond to a variety of stress conditions such as high irradiance. It can aggregate with carotenoids and form rings around the PSI reaction center complexes to aid in photoprotective energy dissipation.

Orange carotenoid protein (OCP) is a water-soluble protein which plays a role in photoprotection in diverse cyanobacteria. It is the only photoactive protein known to use a carotenoid as the photoresponsive chromophore. The protein consists of two domains, with a single keto-carotenoid molecule non-covalently bound between the two domains. It is a very efficient quencher of excitation energy absorbed by the primary light-harvesting antenna complexes of cyanobacteria, the phycobilisomes. The quenching is induced by blue-green light. It is also capable of preventing oxidative damage by directly scavenging singlet oxygen (1O2).
3′-Hydroxyechinenone is a keto-carotenoid pigment found in cyanobacteria and microalgae. Carotenoids belong to a larger class of phytochemicals known as terpenoids. The chemical formula of canthaxanthin is C40H54O2. It is found non-covalently bound in the orange carotenoid protein (OCP), which is a soluble protein involved in photoprotection and non-photochemical quenching of photosynthesis.
Myxoxanthophyll is a carotenoid glycoside pigment present in the photosynthetic apparatus of cyanobacteria. It is named after the word "Myxophyceae", a former term for cyanobacteria. As a monocyclic xanthophyll, it has a yellowish color. It is required for normal cell wall structure and thylakoid organization in the cyanobacterium Synechocystis. The pigment is unusual because it is glycosylated on the 2'-OH rather than the 1'-OH position of the molecule. Myxoxanthophyll was first isolated from Oscillatoria rubenscens in 1936.

Cheryl Ann Kerfeld is an American bioengineer who is Hannah Distinguished Professor at Michigan State University. She holds a joint position at the Lawrence Berkeley National Laboratory. Her research considers bioinformatics, cellular imaging and structural biology.
References
- 1 2 3 Boulay C, Wilson A, D'Haene S, Kirilovsky D (June 2010). "Identification of a protein required for recovery of full antenna capacity in OCP-related photoprotective mechanism in cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 107 (25): 11620–5. doi: 10.1073/pnas.1002912107 . PMC 2895087 . PMID 20534537.
- 1 2 3 Sutter M, Wilson A, Leverenz RL, Lopez-Igual R, Thurotte A, Salmeen AE, Kirilovsky D, Kerfeld CA (June 2013). "Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 110 (24): 10022–7. doi: 10.1073/pnas.1303673110 . PMC 3683793 . PMID 23716688.
- ↑ Gwizdala M, Wilson A, Kirilovsky D (July 2011). "In vitro reconstitution of the cyanobacterial photoprotective mechanism mediated by the Orange Carotenoid Protein in Synechocystis PCC 6803". The Plant Cell. 23 (7): 2631–43. doi:10.1105/tpc.111.086884. PMC 3226224 . PMID 21764991.
- ↑ Gwizdala M, Wilson A, Omairi-Nasser A, Kirilovsky D (March 2013). "Characterization of the Synechocystis PCC 6803 Fluorescence Recovery Protein involved in photoprotection". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1827 (3): 348–54. doi: 10.1016/j.bbabio.2012.11.001 . PMID 23159727.