In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.
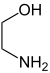
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry.
In organic chemistry, a substituent is one or a group of atoms that replaces atoms, thereby becoming a moiety in the resultant (new) molecule.
In chemistry, an acetylide is a compound that can be viewed as the result of replacing one or both hydrogen atoms of acetylene (ethyne) HC≡CH by metallic or other cations. The term is also used, more loosely, for any compound obtained in the same way from an acetylene derivative RC≡CH, where R is some organic side chain.
In chemistry, the valence or valency of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be two bonds, triple bonds to be three, quadruple bonds to be four, quintuple bonds to be five and sextuple bonds to be six. In most compounds, the valence of hydrogen is 1, of oxygen is 2, of nitrogen is 3, and of carbon is 4. Valence is not to be confused with the related concepts of the coordination number, the oxidation state, or the number of valence electrons for a given atom.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.

Chain-growth polymerization (AE) or chain-growth polymerisation (BE) is a polymerization technique where monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
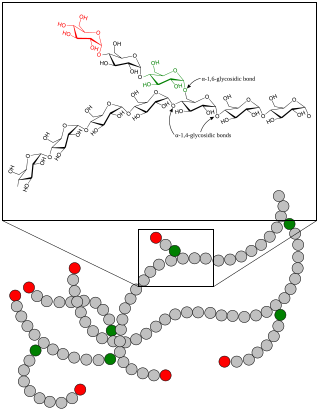
In polymer chemistry, branching is the regular or irregular attachment of side chains to a polymer's backbone chain. It occurs by the replacement of a substituent on a monomer subunit by another covalently-bonded chain of that polymer; or, in the case of a graft copolymer, by a chain of another type. Branched polymers have more compact and symmetrical molecular conformations, and exhibit intra-heterogeneous dynamical behavior with respect to the unbranched polymers. In crosslinking rubber by vulcanization, short sulfur branches link polyisoprene chains into a multiple-branched thermosetting elastomer. Rubber can also be so completely vulcanized that it becomes a rigid solid, so hard it can be used as the bit in a smoking pipe. Polycarbonate chains can be crosslinked to form the hardest, most impact-resistant thermosetting plastic, used in safety glasses.
In polymer chemistry, chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
In organic chemistry, umpolung or polarity inversion is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule.

Carbon nanotube chemistry involves chemical reactions, which are used to modify the properties of carbon nanotubes (CNTs). CNTs can be functionalized to attain desired properties that can be used in a wide variety of applications. The two main methods of CNT functionalization are covalent and non-covalent modifications.

Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).
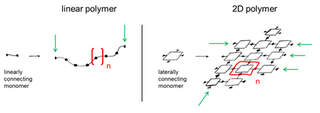
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

In host–guest chemistry, macromolecular cages are a type of macromolecule structurally consisting of a three-dimensional chamber surrounded by a molecular framework. Macromolecular cage architectures come in various sizes ranging from 1-50 nm and have varying topologies as well as functions. They can be synthesized through covalent bonding or self-assembly through non-covalent interactions. Most macromolecular cages that are formed through self-assembly are sensitive to pH, temperature, and solvent polarity.