Related Research Articles
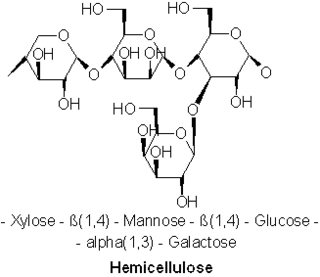
A hemicellulose is one of a number of heteropolymer, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. While cellulose is crystalline, strong, and resistant to hydrolysis, hemicelluloses have random, amorphous structure with little strength. They are easily hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

Polysaccharides, or polycarbohydrates, are the most abundant carbohydrate found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin.
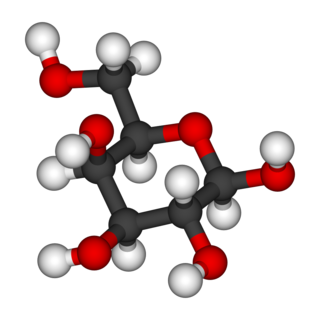
Galactose sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule.

Glycosaminoglycans (GAGs) or mucopolysaccharides are long linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, with the exception of keratan, where in the place of the uronic sugar it has galactose. Because GAGs are highly polar and attract water, they are used in the body as a lubricant or shock absorber. Mucopolysaccharidoses are a group of metabolic disorders in which abnormal accumulations of glycosaminoglycans occur because of enzyme deficiencies.

Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.

Aspergillus is a genus consisting of a few hundred mould species found in various climates worldwide.

Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.
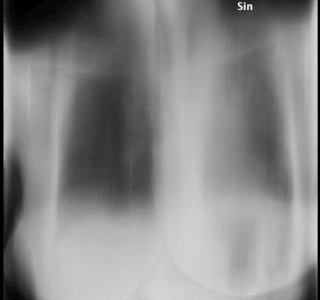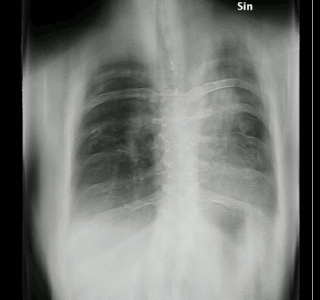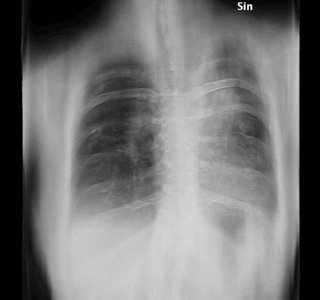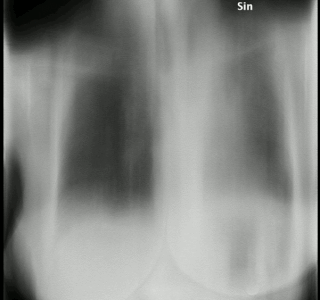
Aspergillosis is a fungal infection of usually the lungs, caused by the genus Aspergillus, a common mold that is breathed in frequently from the air around, but does not usually affect most people. It generally occurs in people with lung diseases such as asthma, cystic fibrosis or tuberculosis, or those who have had a stem cell or organ transplant, and those who cannot fight infection because of medications they take such as steroids and some cancer treatments. Rarely, it can affect skin.

Gliotoxin is a sulfur-containing mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by several species of fungi, especially those of marine origin. It is the most prominent member of the epipolythiopiperazines, a large class of natural products featuring a diketopiperazine with di- or polysulfide linkage. These highly bioactive compounds have been the subject of numerous studies aimed at new therapeutics. Gliotoxin was originally isolated from Gliocladium fimbriatum, and was named accordingly. It is an epipolythiodioxopiperazine metabolite.
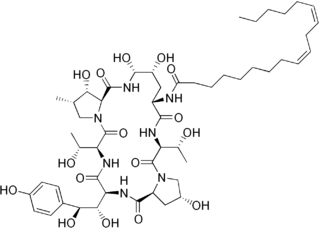
Echinocandins are a class of antifungal drugs that inhibit the synthesis of β-glucan in the fungal cell wall via noncompetitive inhibition of the enzyme 1,3-β glucan synthase. The class has been termed the "penicillin of antifungals," along with the related papulacandins, as their mechanism of action resembles that of penicillin in bacteria. β-glucans are carbohydrate polymers that are cross-linked with other fungal cell wall components, the fungal equivalent to bacterial peptidoglycan. Caspofungin, micafungin, and anidulafungin are semisynthetic echinocandin derivatives with limited clinical use due to their solubility, antifungal spectrum, and pharmacokinetic properties.

UTP—glucose-1-phosphate uridylyltransferase also known as glucose-1-phosphate uridylyltransferase is an enzyme involved in carbohydrate metabolism. It synthesizes UDP-glucose from glucose-1-phosphate and UTP; i.e.,

Galactose epimerase deficiency, also known as GALE deficiency, Galactosemia III and UDP-galactose-4-epimerase deficiency, is a rare, autosomal recessive form of galactosemia associated with a deficiency of the enzyme galactose epimerase.
Galactosyltransferase is a type of glycosyltransferase which catalyzes the transfer of galactose. An example is B-N-acetylglucosaminyl-glycopeptide b-1,4-galactosyltransferase.
1,3-Beta-glucan synthase is a glucosyltransferase enzyme involved in the generation of beta-glucan in fungi. It serves as a pharmacological target for antifungal drugs such as caspofungin, anidulafungin, and micafungin, deemed 1,3-Beta-glucan synthase inhibitors. Under the CAZy classification system, fungi and plant members fall in the glycosyltransferase 48 family (GT48). Some members of the glycosyltransferase 2 family, such as the curdlan synthase CrdS, also has a similar activity.

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.
The gal operon is a prokaryotic operon, which encodes enzymes necessary for galactose metabolism. Repression of gene expression for this operon works via binding of repressor molecules to two operators. These repressors dimerize, creating a loop in the DNA. The loop as well as hindrance from the external operator prevent RNA polymerase from binding to the promoter, and thus prevent transcription. Additionally, since the metabolism of galactose in the cell is involved in both anabolic and catabolic pathways, a novel regulatory system using two promoters for differential repression has been identified and characterized within the context of the gal operon.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.

Epimerox is an experimental broad-spectrum antibiotic compound being developed by scientists at the Rockefeller University and Astex Pharmaceuticals. It is a small molecule inhibitor compound that blocks the activity of the enzyme UDP-N-acetylglucosamine 2-epimerase, an epimerase enzyme that is called 2-epimerase for short.
Polyglucan is any polysaccharide that contains glucan units. Specifically, polyglucan's are a structural polysaccharide. The basic polyglucan unit consists of a long linear chain of several hundred to many thousands D-glucose monomers attached with a type of covalent bond called, glycosidic bonds. The point of attachment is O-glycosidic bonds, where a glycosidic oxygen links the glycoside to the reducing end sugar. Polyglucans naturally occur in the cell walls of bacteria. Bacteria produce this polysaccharide in a cluster near the bacteria's cells. Polyglucan's are a source of beta-glucans. Structurally, beta 1.3-glucans are complex glucose homopolymers binding together in a beta-1,3 configuration.
References
- ↑ Bardalaye, P.C., and Nordin, J.H. (1976). Galactosaminogalactan from cell walls of Aspergillus niger. J Bacteriol 125, 655-669
- ↑ Takada, H., Arakj, Y., Ito, E. (1980). Structure of Polygalactosamine Produced by Aspergillus parasiticus. Biochem. 89, 1265-1274
- ↑ Fontaine, T., Delangle, A., Simenel, C., Coddeville, B., van Vliet, S.J., van Kooyk, Y., Bozza, S., Moretti, S., Schwarz, F., Trichot, C., et al. (2011). Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. In PLoS Pathog, pp. e1002372
- ↑ Gravelat, F.N., Beauvais, A., Liu, H., Lee, M.J., Snarr, B.D., Chen, D., Xu, W., Kravtsov, I., Hoareau, C.M.Q., Vanier, G., et al. (2013). Aspergillus Galactosaminogalactan Mediates Adherence to Host Constituents and Conceals Hyphal β-Glucan from the Immune System. In PLoS Pathog. e.1003575
- ↑ Loussert, C., Schmitt, C., Prevost, M.C., Balloy, V., Fadel, E., Philippe, B., Kauffmann-Lacroix, C., Latge, J.P., and Beauvais, A. (2010). In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol 12, 405-410
- ↑ Robinet, P., Baychelier, F., Fontaine, T., Picard, C., Debre, P., Vieillard, V., Latge, J.P., and Elbim, C. (2014). A Polysaccharide Virulence Factor of a Human Fungal Pathogen Induces Neutrophil Apoptosis via NK Cells. Journal of Immunology. 192(11):5332-42
- ↑ Gresnigt, M.S., Bozza, S., Becker, K.L., Joosten, L.A., Abdollahi-Roodsaz, S., van der Berg, W.B., Dinarello, C.A., Netea, M.G., Fontaine, T., De Luca, A., et al. (2014). A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog 10, e1003936
- ↑ Lee MJ, Gravelat FN, Cerone RP, Baptista SD, Campoli PV, Choe SI, Kravtsov I, Vinogradov E, Creuzenet C, Liu H, Berghuis AM, Latgé JP, Filler SG, Fontaine T, Sheppard DC. Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. J Biol Chem. 2014 Jan 17;289(3):1243-56.