Related Research Articles

Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals including peptides and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of these protein-based therapeutics have also been developed.

In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ligare, which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure.
Neuropeptide Y receptors are a family of receptors belonging to class A G-protein coupled receptors and they are activated by the closely related peptide hormones neuropeptide Y, peptide YY and pancreatic polypeptide. These receptors are involved in the control of a diverse set of behavioral processes including appetite, circadian rhythm, and anxiety.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Lobeline is a piperidine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.

The human muscarinic acetylcholine receptor M5, encoded by the CHRM5 gene, is a member of the G protein-coupled receptor superfamily of integral membrane proteins. It is coupled to Gq protein. Binding of the endogenous ligand acetylcholine to the M5 receptor triggers a number of cellular responses such as adenylate cyclase inhibition, phosphoinositide degradation, and potassium channel modulation. Muscarinic receptors mediate many of the effects of acetylcholine in the central and peripheral nervous system. The clinical implications of this receptor have not been fully explored; however, stimulation of this receptor is known to effectively decrease cyclic AMP levels and downregulate the activity of protein kinase A (PKA).
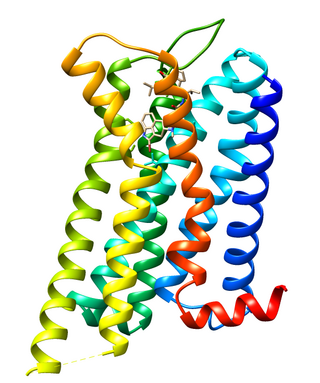
Dopamine receptor D2, also known as D2R, is a protein that, in humans, is encoded by the DRD2 gene. After work from Paul Greengard's lab had suggested that dopamine receptors were the site of action of antipsychotic drugs, several groups, including those of Solomon Snyder and Philip Seeman used a radiolabeled antipsychotic drug to identify what is now known as the dopamine D2 receptor. The dopamine D2 receptor is the main receptor for most antipsychotic drugs. The structure of DRD2 in complex with the atypical antipsychotic risperidone has been determined.

The adenosine A2B receptor, also known as ADORA2B, is a G-protein coupled adenosine receptor, and also denotes the human adenosine A2b receptor gene which encodes it.

G protein-coupled receptor 55 also known as GPR55 is a G protein-coupled receptor that in humans is encoded by the GPR55 gene.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Metabotropic glutamate receptor 3 (mGluR3) is an inhibitory Gi/G0-coupled G-protein coupled receptor (GPCR) generally localized to presynaptic sites of neurons in classical circuits. However, in higher cortical circuits in primates, mGluR3 are localized post-synaptically, where they strengthen rather than weaken synaptic connectivity. In humans, mGluR3 is encoded by the GRM3 gene. Deficits in mGluR3 signaling have been linked to impaired cognition in humans, and to increased risk of schizophrenia, consistent with their expanding role in cortical evolution.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

GABA transporter 1 (GAT1) also known as sodium- and chloride-dependent GABA transporter 1 is a protein that in humans is encoded by the SLC6A1 gene and belongs to the solute carrier 6 (SLC6) family of transporters. It mediates gamma-aminobutyric acid's translocation from the extracellular to intracellular spaces within brain tissue and the central nervous system as a whole.

The γ-hydroxybutyrate (GHB) receptor (GHBR), originally identified as GPR172A, is an excitatory G protein-coupled receptor (GPCR) that binds the neurotransmitter and psychoactive drug γ-hydroxybutyric acid (GHB). As solute carrier family 52 member 2 (SLC52A2), it is also a transporter for riboflavin.
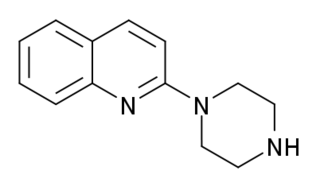
Quipazine is a serotonergic drug of the piperazine group which is used in scientific research. It was originally intended as an antidepressant but never developed for medical use.
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor and the ganglion-type nicotinic receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the autonomic ganglia and adrenal medulla, where activation yields post- and/or presynaptic excitation, mainly by increased Na+ and K+ permeability.
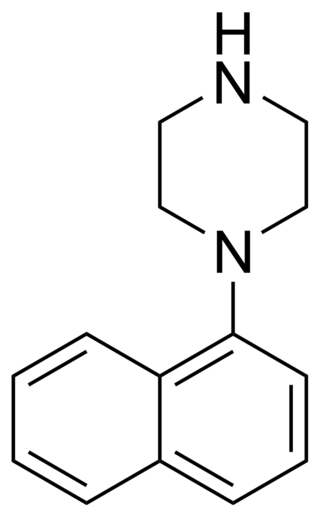
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.
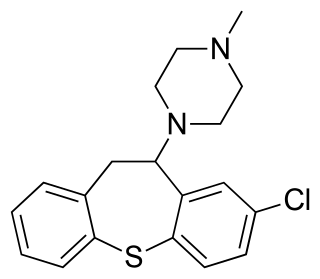
Clorotepine, also known as octoclothepin or octoclothepine, is an antipsychotic of the tricyclic group which was derived from perathiepin in 1965 and marketed in the Czech Republic by Spofa in or around 1971 for the treatment of schizophrenic psychosis.
References
- ↑ Williams, A. J.; Harland, L.; Groth, P.; Pettifer, S.; Chichester, C.; Willighagen, E. L.; Evelo, C. T.; Blomberg, N.; Ecker, G.; Goble, C.; Mons, B. (2012). "Open PHACTS: Semantic interoperability for drug discovery" (PDF). Drug Discovery Today . 17 (21–22): 1188–1198. doi: 10.1016/j.drudis.2012.05.016 . PMID 22683805.
- ↑ His university-hosted CV - "Gerhard Ecker Home". Archived from the original on 2011-09-18. Retrieved 2011-12-07. - this cross-references with his publications on Google Scholar: https://scholar.google.com/scholar?as_q=&as_sauthors=Gerhard+Ecker+Fleischhacker+Noe
- ↑ Bio from wiley.com - for Transporters as Drug Carriers: Structure, Function, Substrates, Volume 44 - http://onlinelibrary.wiley.com/book/10.1002/9783527627424/homepage/AuthorBiography.html
- ↑ Molecular Informatics
- ↑ European Federation for Medicinal Chemistry
- ↑ Jabeen, I.; Wetwitayaklung, P.; Klepsch, F.; Parveen, Z.; Chiba, P.; Ecker, G. F. (2011). "Probing the stereoselectivity of P-glycoprotein—synthesis, biological activity and ligand docking studies of a set of enantiopure benzopyrano\3,4-b]\1,4]oxazines". Chemical Communications. 47 (9): 2586–2588. doi:10.1039/C0CC03075A. PMID 21173990. S2CID 205756984.
- ↑ Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G. F.; Faller, B.; Fischer, H.; Gerebtzoff, G. G.; Lennernaes, H.; Senner, F. (2010). "Coexistence of passive and carrier-mediated processes in drug transport". Nature Reviews Drug Discovery. 9 (8): 597–614. doi:10.1038/nrd3187. PMID 20671764. S2CID 34829942.
- ↑ Klepsch, F.; Ecker, G. F. (2010). "Impact of the Recent Mouse P-Glycoprotein Structure for Structure-Based Ligand Design". Molecular Informatics. 29 (4): 276–86. doi:10.1002/minf.201000017. PMC 6422301 . PMID 27463054.
- ↑ Parveen, Z.; Stockner, T.; Bentele, C.; Pferschy, S.; Kraupp, M.; Freissmuth, M.; Ecker, G. F.; Chiba, P. (2010). "Molecular Dissection of Dual Pseudosymmetric Solute Translocation Pathways in Human P-Glycoprotein". Molecular Pharmacology. 79 (3): 443–452. doi:10.1124/mol.110.067611. PMC 6422312 . PMID 21177413.
- ↑ Thai, K. M.; Windisch, A.; Stork, D.; Weinzinger, A.; Schiesaro, A.; Guy, R. H.; Timin, E. N.; Hering, S.; Ecker, G. F. (2010). "The hERG Potassium Channel and Drug Trapping: Insight from Docking Studies with Propafenone Derivatives". ChemMedChem. 5 (3): 436–442. doi:10.1002/cmdc.200900374. PMID 20146282. S2CID 23597808.
- ↑ Sarker, S.; Weissensteiner, R.; Steiner, I.; Sitte, H. H.; Ecker, G. F.; Freissmuth, M.; Sucic, S. (2010). "The High-Affinity Binding Site for Tricyclic Antidepressants Resides in the Outer Vestibule of the Serotonin Transporter". Molecular Pharmacology. 78 (6): 1026–1035. doi:10.1124/mol.110.067538. PMC 4513247 . PMID 20829432.
- ↑ Khom, S.; Strommer, B.; Ramharter, J.; Schwarz, T.; Schwarzer, C.; Erker, T.; Ecker, G. F.; Mulzer, J.; Hering, S. (2010). "Valerenic acid derivatives as novel subunit-selective GABAA receptor ligands -in vitro and in vivo characterization". British Journal of Pharmacology. 161 (1): 65–78. doi:10.1111/j.1476-5381.2010.00865.x. PMC 2962817 . PMID 20718740.
- ↑ Search Results for author Ecker GF on PubMed .