Related Research Articles
Cell biology is a branch of biology that studies the structure, function, and behavior of cells. All living organisms are made of cells. A cell is the basic unit of life that is responsible for the living and functioning of organisms. Cell biology is the study of the structural and functional units of cells. Cell biology encompasses both prokaryotic and eukaryotic cells and has many subtopics which may include the study of cell metabolism, cell communication, cell cycle, biochemistry, and cell composition. The study of cells is performed using several microscopy techniques, cell culture, and cell fractionation. These have allowed for and are currently being used for discoveries and research pertaining to how cells function, ultimately giving insight into understanding larger organisms. Knowing the components of cells and how cells work is fundamental to all biological sciences while also being essential for research in biomedical fields such as cancer, and other diseases. Research in cell biology is interconnected to other fields such as genetics, molecular genetics, molecular biology, medical microbiology, immunology, and cytochemistry.

An electron microscope is a microscope that uses a beam of electrons as a source of illumination. They use electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing them to produce magnified images or electron diffraction patterns. As the wavelength of an electron can be up to 100,000 times smaller than that of visible light, electron microscopes have a much higher resolution of about 0.1 nm, which compares to about 200 nm for light microscopes. Electron microscope may refer to:

Integrins are transmembrane receptors that help cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

Structural biology, as defined by the Journal of Structural Biology, deals with structural analysis of living material at every level of organization.

The Max Planck Institute of Biochemistry is a research institute of the Max Planck Society located in Martinsried, a suburb of Munich. The institute was founded in 1973 by the merger of three formerly independent institutes: the Max Planck Institute of Biochemistry, the Max Planck Institute of Protein and Leather Research, and the Max Planck Institute of Cell Chemistry.

Transmission electron cryomicroscopy (CryoTEM), commonly known as cryo-EM, is a form of cryogenic electron microscopy, more specifically a type of transmission electron microscopy (TEM) where the sample is studied at cryogenic temperatures. Cryo-EM, specifically 3-dimensional electron microscopy (3DEM), is gaining popularity in structural biology.

Cryogenic electron tomography (cryoET) is an imaging technique used to reconstruct high-resolution (~1–4 nm) three-dimensional volumes of samples, often biological macromolecules and cells. cryoET is a specialized application of transmission electron cryomicroscopy (CryoTEM) in which samples are imaged as they are tilted, resulting in a series of 2D images that can be combined to produce a 3D reconstruction, similar to a CT scan of the human body. In contrast to other electron tomography techniques, samples are imaged under cryogenic conditions. For cellular material, the structure is immobilized in non-crystalline, vitreous ice, allowing them to be imaged without dehydration or chemical fixation, which would otherwise disrupt or distort biological structures.
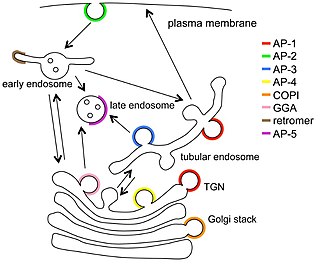
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned.

Richard Henderson is a British molecular biologist and biophysicist and pioneer in the field of electron microscopy of biological molecules. Henderson shared the Nobel Prize in Chemistry in 2017 with Jacques Dubochet and Joachim Frank. "Thanks to his work, we can look at individual atoms of living nature, thanks to cryo-electron microscopes we can see details without destroying samples, and for this he won the Nobel Prize in Chemistry."

Eva Nogales is a Spanish-American biophysicist at the Lawrence Berkeley National Laboratory and a professor at the University of California, Berkeley, where she served as head of the Division of Biochemistry, Biophysics and Structural Biology of the Department of Molecular and Cell Biology (2015–2020). She is a Howard Hughes Medical Institute investigator.
The NAS Award in Molecular Biology is awarded by the U.S. National Academy of Sciences "for recent notable discovery in molecular biology by a young scientist who is a citizen of the United States." It has been awarded annually since its inception in 1962.
The Colworth Medal is awarded annually by the Biochemical Society to an outstanding research biochemist under the age of 35 and working mainly in the United Kingdom. The award is one of the most prestigious recognitions for young scientists in the UK, and was established by Tony James FRS at Unilever Research and Henry Arnstein of the Biochemical Society and takes its name from a Unilever research laboratory near Bedford in the UK, Colworth House.

In molecular biology, the term macromolecular assembly (MA) refers to massive chemical structures such as viruses and non-biologic nanoparticles, cellular organelles and membranes and ribosomes, etc. that are complex mixtures of polypeptide, polynucleotide, polysaccharide or other polymeric macromolecules. They are generally of more than one of these types, and the mixtures are defined spatially, and with regard to their underlying chemical composition and structure. Macromolecules are found in living and nonliving things, and are composed of many hundreds or thousands of atoms held together by covalent bonds; they are often characterized by repeating units. Assemblies of these can likewise be biologic or non-biologic, though the MA term is more commonly applied in biology, and the term supramolecular assembly is more often applied in non-biologic contexts. MAs of macromolecules are held in their defined forms by non-covalent intermolecular interactions, and can be in either non-repeating structures, or in repeating linear, circular, spiral, or other patterns. The process by which MAs are formed has been termed molecular self-assembly, a term especially applied in non-biologic contexts. A wide variety of physical/biophysical, chemical/biochemical, and computational methods exist for the study of MA; given the scale of MAs, efforts to elaborate their composition and structure and discern mechanisms underlying their functions are at the forefront of modern structure science.

Maya Schuldiner is an Israeli molecular geneticist, a full professor at the Faculty of Biochemistry in the Weizmann Institute of Science, who serves as the chair of its scientific council. Her research aims to achieve a mechanistic understanding of the basic functions underlying intracellular organization.

Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample solution is applied to a grid-mesh and plunge-frozen in liquid ethane or a mixture of liquid ethane and propane. While development of the technique began in the 1970s, recent advances in detector technology and software algorithms have allowed for the determination of biomolecular structures at near-atomic resolution. This has attracted wide attention to the approach as an alternative to X-ray crystallography or NMR spectroscopy for macromolecular structure determination without the need for crystallization.
Halperin-Birk syndrome (HLBKS) is a rare autosomal recessive neurodevelopmental disorder caused by a null mutation in the SEC31A gene. Signs and symptoms include intrauterine growth retardation, marked developmental delay, spastic quadriplegia with profound contractures, dysmorphism, and optic nerve atrophy with no eye fixation. Brain MRI demonstrated microcephaly and agenesis of the corpus callosum.

Deborah F. Kelly is an American biomedical engineer who is a professor at Pennsylvania State University. Her research makes use of cryogenic electron microscopy to better understand human development and disease. She was elected President of the Microscopy Society of America in 2022.

Tanmay A. M. Bharat is a programme leader in the Structural Studies Division of the MRC Laboratory of Molecular Biology. He and his group use electron tomography, together with several structural and cell biology methods to study the cell surfaces of bacteria and archaea. His work has increased the understanding of how surface molecules help in the formation of multicellular communities of prokaryotes, examples of which include biofilms and microbiomes. He has been awarded several prizes and fellowships for his work.

Virus crystallisation is the re-arrangement of viral components into solid crystal particles. The crystals are composed of thousands of inactive forms of a particular virus arranged in the shape of a prism. The inactive nature of virus crystals provide advantages for immunologists to effectively analyze the structure and function behind viruses. Understanding of such characteristics have been enhanced thanks to the enhancement and diversity in crystallisation technologies. Virus crystals have a deep history of being widely applied in epidemiology and virology, and still to this day remains a catalyst for studying viral patterns to mitigate potential disease outbreaks.
References
- 1 2 3 4 5 "Introducing... Giulia Zanetti". Crick. 9 July 2024. Retrieved 2024-07-26.
- ↑ "The Colworth Medal". www.biochemistry.org. Retrieved 2024-07-28.
- ↑ "Dr. Giulia Zanetti awarded Biochemical Society Colworth Medal – ISMB". www.ismb.lon.ac.uk. Retrieved 2024-07-28.
- ↑ "Giulia Zanetti". Crick. 9 July 2024. Retrieved 2024-07-26.
- ↑ "COPII coat | Zanetti lab | London". research-home. Retrieved 2024-07-26.