
The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

Rimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the severity of influenza. Rimantadine can mitigate symptoms, including fever. Both rimantadine and the similar drug amantadine are derivates of adamantane. Rimantadine is found to be more effective than amantadine because when used the patient displays fewer symptoms. Rimantadine was approved by the Food and Drug Administration (FDA) in 1994.
Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended because of widespread drug resistance. It is also used for a variety of other uses. The drug is taken by mouth.
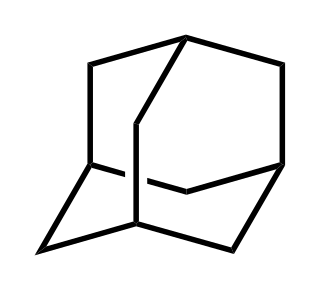
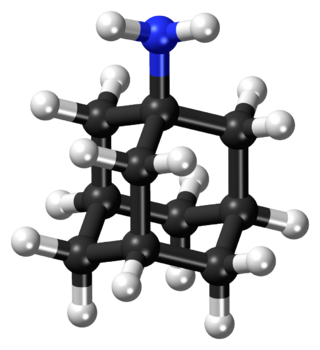
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.
Memantine, sold under the brand name Axura among others, is a medication used to slow the progression of moderate-to-severe Alzheimer's disease. It is taken by mouth.

CX717 is an ampakine compound created by Christopher Marrs and Gary Rogers in 1996 at Cortex Pharmaceuticals. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.
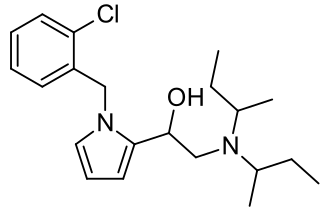
Viminol is an opioid analgesic developed by a team at the drug company Zambon in the 1960s. Viminol is based on the α-pyrryl-2-aminoethanol structure, unlike any other class of opioids.

Neramexane is a drug related to memantine, which acts as an NMDA antagonist and has neuroprotective effects. It is being developed for various possible applications, including treatment of tinnitus, Alzheimer's disease, drug addiction and as an analgesic. Animal studies have also suggested antidepressant and nootropic actions so that this drug may be used for a wide range of potential applications. It also acts as a nicotinic acetylcholine receptor antagonist.

Bifemelane (INN) (Alnert, Celeport), or bifemelane hydrochloride (JAN), also known as 4-(O-benzylphenoxy)-N-methylbutylamine, is an antidepressant and cerebral activator that was widely used in the treatment of cerebral infarction patients with depressive symptoms in Japan, and in the treatment of senile dementia as well. It also appears to be useful in the treatment of glaucoma. It has been discontinued in Japan since 1998, when it was removed from the market reportedly for lack of effectiveness.

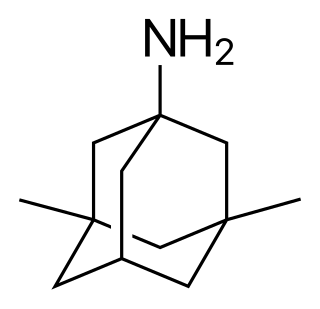
Bromantane, sold under the brand name Ladasten, is an atypical central nervous system (CNS) stimulant and anxiolytic drug of the adamantane family that is related to amantadine and memantine. Medically, it is approved in Russia for the treatment of neurasthenia. Although the effects of bromantane have been determined to be dependent on the dopaminergic and possibly serotonergic neurotransmitter systems, its exact mechanism of action is unknown, and is distinct in its properties relative to typical stimulants such as amphetamine. Bromantane has sometimes been described as an actoprotector.
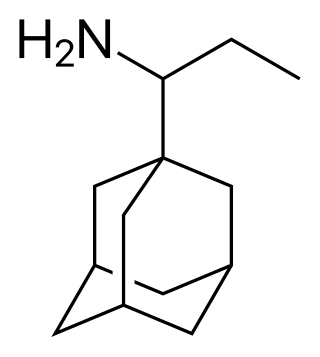
Adapromine is an antiviral drug of the adamantane group related to amantadine (1-aminoadamantane), rimantadine, and memantine (1-amino-3,5-dimethyladamantane) that is marketed in Russia for the treatment and prevention of influenza. It is an alkyl analogue of rimantadine and is similar to rimantadine in its antiviral activity but possesses a broader spectrum of action, being effective against influenza viruses of both type A and B. Strains of type A influenza virus with resistance to adapromine and rimantadine and the related drug deitiforine were encountered in Mongolia and the Soviet Union in the 1980s.

Cinazepam is an atypical benzodiazepine derivative. It produces pronounced hypnotic, sedative, and anxiolytic effects with minimal myorelaxant side effects. In addition, unlike many other benzodiazepine and nonbenzodiazepine hypnotics such as diazepam, flunitrazepam, and zopiclone, cinazepam does not violate sleep architecture, and the continuity of slow-wave sleep and REM sleep are proportionally increased. As such, cinazepam produces a sleep state close to physiological, and for that reason, may be advantageous compared to other, related drugs in the treatment of insomnia and other sleep disorders.
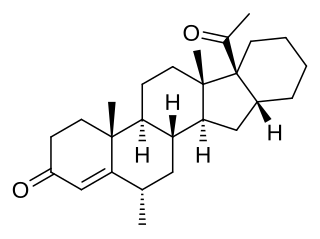
Mecigestone, also known as pentarane B, as well as 6α-methyl-16α,17α-cyclohexanoprogesterone, 6α-methylcyclohexano[1',2';16,17]pregn-4-ene-3,20-dione, or 17α-acetyl-6α-methyl-16β,24-cyclo-21-norchol-4-en-3-one, is a steroidal progestin that was developed by the Zelinskii Institute of Organic Chemistry of the Russian Academy of Sciences and has been proposed for clinical use as a progestogen but has not been marketed. It is the 6α-methylated analogue of pentarane A, which is also known as D'6-pentarane or pregna-D'6-pentarane.
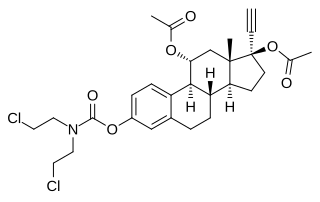
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent which was developed for the treatment of breast cancer but was never marketed.

Cortifen, also known as cortiphen or kortifen, as well as fencoron, is a synthetic glucocorticoid corticosteroid and cytostatic antineoplastic agent which was developed in Russia for potential treatment of tumors. It is a hydrophobic chlorphenacyl nitrogen mustard ester of 11-deoxycortisol (cortodoxone).
Actoprotectors or synthetic adaptogens are compounds that enhance an organism's resilience to physical stress without increasing heat output. Actoprotectors are distinct from other performance-enhancing substances in that they increase physical and psychological resilience via non-exhaustive action. The term "actoprotector" is used to describe synthetic and isolated compounds possessing adaptogenic properties. By contrast, the term "adaptogen" is most often use to describe a natural herb as a whole, which can contain hundreds if not thousands of biologically active components.

Ethylestradiol, or 17α-ethylestradiol, also known as 17α-ethylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrogen which was never marketed. It occurs as an active metabolite of the anabolic steroids norethandrolone and ethylestrenol formed via aromatase and is believed to be responsible for the estrogenic effects of norethandrolone and ethylestrenol. The 3-methyl ether of ethylestradiol has been used as an intermediate in the synthesis of certain 19-nortestosterone anabolic steroids.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.

Dopamantine is an antiparkinsonian drug of the adamantane group that developed for treatment of Parkinson's disease but was never marketed. It was developed and studied in the 1970s and was said to have reached early clinical trials.

Hemantane, or hymantane, also known as N-(2-adamantyl)hexamethyleneimine, is an experimental antiparkinsonian agent of the adamantane family that was never marketed. It was developed and studied in Russia.



















