Related Research Articles

Gluten is a structural protein naturally found in certain cereal grains. The term gluten usually refers to the combination of prolamin and glutelin proteins that naturally occur in many cereal grains, and which can trigger celiac disease in some people. The types of grains that contain gluten include all species of wheat, and barley, rye, and some cultivars of oat; moreover, cross hybrids of any of these cereal grains also contain gluten, e.g. triticale. Gluten makes up 75–85% of the total protein in bread wheat.

Coeliac disease or celiac disease is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barley. Classic symptoms include gastrointestinal problems such as chronic diarrhoea, abdominal distention, malabsorption, loss of appetite, and among children failure to grow normally. Non-classic symptoms are more common, especially in people older than two years. There may be mild or absent gastrointestinal symptoms, a wide number of symptoms involving any part of the body, or no obvious symptoms. Coeliac disease was first described in childhood; however, it may develop at any age. It is associated with other autoimmune diseases, such as Type 1 diabetes mellitus and Hashimoto's thyroiditis, among others.

Irritable bowel syndrome (IBS) is a "disorder of gut-brain interaction" characterized by a group of symptoms that commonly include abdominal pain, abdominal bloating and changes in the consistency of bowel movements. These symptoms may occur over a long time, sometimes for years. IBS can negatively affect quality of life and may result in missed school or work or reduced productivity at work. Disorders such as anxiety, major depression, and chronic fatigue syndrome are common among people with IBS.

A gluten-free diet (GFD) is a nutritional plan that strictly excludes gluten, which is a mixture of prolamin proteins found in wheat, as well as barley, rye, and oats. The inclusion of oats in a gluten-free diet remains controversial, and may depend on the oat cultivar and the frequent cross-contamination with other gluten-containing cereals.
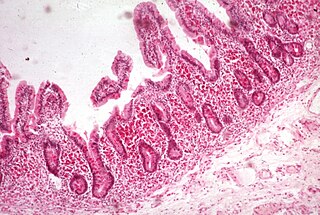
Malabsorption is a state arising from abnormality in absorption of food nutrients across the gastrointestinal (GI) tract. Impairment can be of single or multiple nutrients depending on the abnormality. This may lead to malnutrition and a variety of anaemias.
Food intolerance is a detrimental reaction, often delayed, to a food, beverage, food additive, or compound found in foods that produces symptoms in one or more body organs and systems, but generally refers to reactions other than food allergy. Food hypersensitivity is used to refer broadly to both food intolerances and food allergies.

Gliadin is a class of proteins present in wheat and several other cereals within the grass genus Triticum. Gliadins, which are a component of gluten, are essential for giving bread the ability to rise properly during baking. Gliadins and glutenins are the two main components of the gluten fraction of the wheat seed. This gluten is found in products such as wheat flour. Gluten is split about evenly between the gliadins and glutenins, although there are variations found in different sources.

Eosinophilic esophagitis (EoE) is an allergic inflammatory condition of the esophagus that involves eosinophils, a type of white blood cell. In healthy individuals, the esophagus is typically devoid of eosinophils. In EoE, eosinophils migrate to the esophagus in large numbers. When a trigger food is eaten, the eosinophils contribute to tissue damage and inflammation. Symptoms include swallowing difficulty, food impaction, vomiting, and heartburn.

Gluten-related disorders is the term for the diseases triggered by gluten, including celiac disease (CD), non-celiac gluten sensitivity (NCGS), gluten ataxia, dermatitis herpetiformis (DH) and wheat allergy. The umbrella category has also been referred to as gluten intolerance, though a multi-disciplinary physician-led study, based in part on the 2011 International Coeliac Disease Symposium, concluded that the use of this term should be avoided due to a lack of specificity.
Gluten-sensitive enteropathy–associated conditions are comorbidities or complications of gluten-related gastrointestinal distress. GSE has key symptoms typically restricted to the bowel and associated tissues; however, there are a wide variety of associated conditions. These include bowel disorders, eosinophilic gastroenteritis and increase with coeliac disease (CD) severity. With some early onset and a large percentage of late onset disease, other disorders appear prior to the coeliac diagnosis or allergic-like responses markedly increased in GSE. Many of these disorders persist on a strict gluten-free diet, and are thus independent of coeliac disease after triggering. For example, autoimmune thyroiditis is a common finding with GSE.
Anti-gliadin antibodies are produced in response to gliadin, a prolamin found in wheat. In bread wheat it is encoded by three different alleles, AA, BB, and DD. These alleles can produce slightly different gliadins, which can cause the body to produce different antibodies. Some of these antibodies can detect proteins in specific grass taxa such as Triticeae, while others react sporadically with certain species in those taxa, or over many taxonomically defined grass tribes.
Anti-transglutaminase antibodies (ATA) are autoantibodies against the transglutaminase protein. Detection is considered abnormal, and may indicate one of several conditions.

Enteropathy-associated T-cell lymphoma (EATL), previously termed enteropathy-associated T-cell lymphoma, type I and at one time termed enteropathy-type T-cell lymphoma (ETTL), is a complication of coeliac disease in which a malignant T-cell lymphoma develops in areas of the small intestine affected by the disease's intense inflammation. While a relatively rare disease, it is the most common type of primary gastrointestinal T-cell lymphoma.
Oat sensitivity represents a sensitivity to the proteins found in oats, Avena sativa. Sensitivity to oats can manifest as a result of allergy to oat seed storage proteins either inhaled or ingested. A more complex condition affects individuals who have gluten-sensitive enteropathy in which there is an autoimmune response to avenin, the glutinous protein in oats similar to the gluten within wheat. Sensitivity to oat foods can also result from their frequent contamination by wheat, barley, or rye particles.
The immunochemistry of Triticeae glutens is important in several inflammatory diseases. It can be subdivided into innate responses, class II mediated presentation, class I mediated stimulation of killer cells, and antibody recognition. The responses to gluten proteins and polypeptide regions differs according to the type of gluten sensitivity. The response is also dependent on the genetic makeup of the human leukocyte antigen genes. In gluten sensitive enteropathy, there are four types of recognition, innate immunity, HLA-DQ, and antibody recognition of gliadin and transglutaminase. With idiopathic gluten sensitivity only antibody recognition to gliadin has been resolved. In wheat allergy, the response pathways are mediated through IgE against other wheat proteins and other forms of gliadin.

Dermatitis herpetiformis (DH) is a chronic autoimmune blistering skin condition, characterised by intensely itchy blisters filled with a watery fluid. DH is a cutaneous manifestation of coeliac disease, although the exact causal mechanism is not known. DH is neither related to nor caused by herpes virus; the name means that it is a skin inflammation having an appearance similar to herpes.
FODMAPs or fermentable oligosaccharides, disaccharides, monosaccharides, and polyols are short-chain carbohydrates that are poorly absorbed in the small intestine and ferment in the colon. They include short-chain oligosaccharide polymers of fructose (fructans) and galactooligosaccharides, disaccharides (lactose), monosaccharides (fructose), and sugar alcohols (polyols), such as sorbitol, mannitol, xylitol, and maltitol. Most FODMAPs are naturally present in food and the human diet, but the polyols may be added artificially in commercially prepared foods and beverages.
Non-celiac gluten sensitivity (NCGS) or gluten sensitivity is a controversial disorder which can cause both gastrointestinal and other problems.
Duodenal lymphocytosis, sometimes called lymphocytic duodenitis, lymphocytic duodenosis, or duodenal intraepithelial lymphocytosis, is a condition where an increased number of intra-epithelial lymphocytes is seen in biopsies of the duodenal mucosa when these are examined microscopically. This form of lymphocytosis is often a feature of coeliac disease but may be found in other disorders.

Carlo Catassi is an Italian gastroenterologist, epidemiologist, and researcher. He is known for international studies on the epidemiology of celiac disease. Currently, he is Head of the Department of Pediatrics at the Università Politecnica delle Marche, Ancona, Italy, and Visiting Scientist at Massachusetts General Hospital in Boston, Massachusetts, United States. From 2013 to 2016, he served as President of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP). His research had included contributions to understanding the clinical spectrum of celiac disease and other gluten-related disorders.
References
- 1 2 3 4 Volta U, Caio G, De Giorgio R, Henriksen C, Skodje G, Lundin KE (Jun 2015). "Non-celiac gluten sensitivity: a work-in-progress entity in the spectrum of wheat-related disorders". Best Pract Res Clin Gastroenterol. 29 (3): 477–91. doi:10.1016/j.bpg.2015.04.006. PMID 26060112.
- 1 2 3 4 5 Rostom A, Murray JA, Kagnoff MF (Dec 2006). "American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease". Gastroenterology (Review). 131 (6): 1981–2002. doi: 10.1053/j.gastro.2006.10.004 . PMID 17087937.
- 1 2 3 4 5 Elli L, Branchi F, Tomba C, Villalta D, Norsa L, Ferretti F, Roncoroni L, Bardella MT (Jun 2015). "Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity". World J Gastroenterol (Review). 21 (23): 7110–9. doi: 10.3748/wjg.v21.i23.7110 . PMC 4476872 . PMID 26109797.
- 1 2 3 Ontiveros N, Hardy MY, Cabrera-Chavez F (2015). "Assessing of Celiac Disease and Nonceliac Gluten Sensitivity". Gastroenterology Research and Practice (Review). 2015: 1–13. doi: 10.1155/2015/723954 . PMC 4429206 . PMID 26064097.
- 1 2 3 4 Aziz I, Hadjivassiliou M, Sanders DS (Sep 2015). "The spectrum of noncoeliac gluten sensitivity". Nat Rev Gastroenterol Hepatol (Review). 12 (9): 516–26. doi:10.1038/nrgastro.2015.107. PMID 26122473. S2CID 2867448.
- 1 2 Denise Faustman (13 March 2014). The Value of BCG and TNF in Autoimmunity. Elsevier Science. p. 130. ISBN 978-0-12-800461-6.
- ↑ Green PH, Lebwohl B, Greywoode R (May 2015). "Celiac disease". J Allergy Clin Immunol (Review). 135 (5): 1099–106. doi:10.1016/j.jaci.2015.01.044. PMID 25956012. S2CID 21552589.
- ↑ van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE (2010). "Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review". JAMA . 303 (17): 1738–46. doi:10.1001/jama.2010.549. PMID 20442390.
Most studies used similar histological criteria for diagnosing celiac disease (Marsh grade ≥III), but the level of damage may vary across populations. Only 4 studies presented the proportion of patients in whom only partial villous atrophy was found (Marsh grade of IIIA), which ranged from 4% to 100%. The presence of positive serum antibodies has been shown to correlate with the degree of villous atrophy, and patients with celiac disease who have less severe histological damage may have seronegative findings. This could be important, especially in primary care, in which levels of mucosal damage may be lower, and consequently, more patients with celiac disease may be missed.
- ↑ Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Mäki M, Ribes-Koninckx C, Ventura A, Zimmer KP, ESPGHAN Working Group on Coeliac Disease Diagnosis, ESPGHAN Gastroenterology Committee, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (Jan 2012). "European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease". Journal of Pediatric Gastroenterology and Nutrition (Practice Guideline). 54 (1): 136–160. doi: 10.1097/MPG.0b013e31821a23d0 . PMID 22197856. S2CID 15029283.
- 1 2 Vriezinga SL, Schweizer JJ, Koning F, Mearin ML (Sep 2015). "Coeliac disease and gluten-related disorders in childhood". Nature Reviews. Gastroenterology & Hepatology (Review). 12 (9): 527–36. doi:10.1038/nrgastro.2015.98. PMID 26100369. S2CID 2023530.
- 1 2 Lundin KEA, Alaedini A, Non-celiac Gluten Sensitivity. In: Benjamin Lebwohl; Peter H. R. Green (1 November 2012). Celiac Disease, An Issue of Gastrointestinal Endoscopy Clinics. Elsevier Health Sciences. ISBN 978-1-4557-4735-1.
- ↑ Maglione, Margaret A.; Okunogbe, Adeyemi; Ewing, Brett; Grant, Sean; Newberry, Sydne J.; Motala, Aneesa; Shanman, Roberta; Mejia, Nelly; Arifkhanova, Aziza (January 2016). "Table 16, Length of gluten challenge". www.ncbi.nlm.nih.gov. Retrieved 2024-02-19.
- ↑ Volta, Umberto; Caio, Giacomo; Tovoli, Francesco; De Giorgio, Roberto (2013). "Non-celiac gluten sensitivity: an emerging syndrome with many unsettled issues". Italian Journal of Medicine. 8 (4): 225. doi: 10.4081/itjm.2013.461 . ISSN 1877-9352.
- ↑ Caio, Giacomo; Volta, Umberto; Tovoli, Francesco; De Giorgio, Roberto (2014). "Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity". BMC Gastroenterology. 14 (1): 26. doi: 10.1186/1471-230X-14-26 . ISSN 1471-230X. PMC 3926852 . PMID 24524388.
- ↑ H. A. Harfi; F. B. Stapleton; William Oh; H. Nazer; R. J. Whitley (10 January 2012). Textbook of Clinical Pediatrics. Springer Science & Business Media. p. 1899. ISBN 978-3-642-02202-9.