Related Research Articles

Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.

Lipid-anchored proteins are proteins located on the surface of the cell membrane that are covalently attached to lipids embedded within the cell membrane. These proteins insert and assume a place in the bilayer structure of the membrane alongside the similar fatty acid tails. The lipid-anchored protein can be located on either side of the cell membrane. Thus, the lipid serves to anchor the protein to the cell membrane. They are a type of proteolipids.
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule in order to form a glycoconjugate. In biology, glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation may refer to a non-enzymatic reaction.
Glycosylphosphatidylinositol or glycophosphatidylinositol (GPI) is a phosphoglyceride that can be attached to the C-terminus of a protein during posttranslational modification. The resulting GPI-anchored proteins play key roles in a wide variety of biological processes. GPI is composed of a phosphatidylinositol group linked through a carbohydrate-containing linker and via an ethanolamine phosphate (EtNP) bridge to the C-terminal amino acid of a mature protein. The two fatty acids within the hydrophobic phosphatidyl-inositol group anchor the protein to the cell membrane.
In enzymology, a N-acetylglucosaminylphosphatidylinositol deacetylase (EC 3.5.1.89) is an enzyme that catalyzes the chemical reaction

Phosphatidylinositol-glycan-specific phospholipase D is an enzyme that in humans is encoded by the GPLD1 gene.

GPI transamidase component PIG-T is an enzyme that in humans is encoded by the PIGT gene.

GPI-anchor transamidase is an enzyme that in humans is encoded by the PIGK gene.

Glycosylphosphatidylinositol anchor attachment 1 protein is a protein that in humans is encoded by the GPAA1 gene.

Phosphatidylinositol N-acetylglucosaminyltransferase subunit Q is an enzyme that in humans is encoded by the PIGQ gene.

Phosphatidylinositol-glycan biosynthesis class F protein is a protein that in humans is encoded by the PIGF gene.

GPI transamidase component PIG-S is an enzyme that in humans is encoded by the PIGS gene. This gene encodes a protein that is involved in GPI-anchor biosynthesis.

Phosphatidylinositol glycan anchor biosynthesis class U protein is a protein that in humans is encoded by the PIGU gene.

GPI mannosyltransferase 3 is an enzyme that in humans is encoded by the PIGB gene.

Phosphatidylinositol N-acetylglucosaminyltransferase subunit H is an enzyme that in humans is encoded by the PIGH gene. The PIGH gene is located on the reverse strand of chromosome 14 in humans, and is neighbored by TMEM229B.
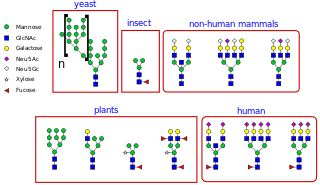
N-linked glycosylation is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan.

dolichyl-phosphate mannosyltransferase polypeptide 3, also known as DPM3, is a human gene.

Dolichol phosphate-mannose biosynthesis regulatory protein is a protein that in humans is encoded by the DPM2 gene.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.

Phosphatidylinositol glycan anchor biosynthesis, class N is a protein that in humans is encoded by the PIGN gene.
References
- ↑ Kobayashi T. et al. (1997) The presence of GPI-linked protein(s) in an archaeobacterium, Sulfolobus acidocaldarius, closely related to eukaryotes. Biochim Biophys Acta. 1334, 1-4.
- ↑ Nosjean O. et al. (1997) Mammalian GPI proteins: Sorting, membrane residence and functions. Biochim Biophys Acta. 1331, 153-86.
- ↑ Thomas J. R. et al. (1990) Structure, biosynthesis, and function of glycosylphosphatidylinositols. Biochemistry. 29, 5413-22.
- ↑ Ikezawa H. (2002) Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol Pharm Bull. 25, 409-17.
- ↑ Brewis I. A. et al. (1995) Structures of the glycosyl-phosphatidylinositol anchors of porcine and human renal membrane dipeptidase. Comprehensive structural studies on the porcine anchor and interspecies comparison of the glycan core structures. J Biol Chem. 270, 22946-56.
- ↑ Low M. G. (1989) Glycosyl-phosphatidylinositol: A versatile anchor for cell surface proteins. FASEB J. 3, 1600-8.
- ↑ Low M. G. and Saltiel A. R. (1988) Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 239, 268-75.
- ↑ Vainauskas S. and Menon A. K. (2006) Ethanolamine phosphate linked to the first mannose residue of glycosylphosphatidylinositol (GPI) lipids is a major feature of the GPI structure that is recognized by human GPI transamidase. J Biol Chem. 281, 38358-64.
- ↑ Menon A. K. et al. (1993) Phosphatidylethanolamine is the donor of the terminal phosphoethanolamine group in trypanosome glycosylphosphatidylinositols. EMBO J. 12, 1907-14.
- ↑ Menon A. K. et al. (1990) Biosynthesis of glycosyl-phosphatidylinositol lipids in Trypanosoma brucei: Involvement of mannosyl-phosphoryldolichol as the mannose donor. EMBO J. 9, 4249-58.
- ↑ Menon A. K. and Stevens V. L. (1992) Phosphatidylethanolamine is the donor of the ethanolamine residue linking a glycosylphosphatidylinositol anchor to protein. J Biol Chem. 267, 15277-80.
- ↑ Orlean P. (1990) Dolichol phosphate mannose synthase is required in vivo for glycosyl-phosphatidylinositol membrane anchoring, O mannosylation, and N glycosylation of protein in saccharomyces cerevisiae. Mol Cell Biol. 10, 5796-805.
- ↑ Imhof I. et al. (2000) Phosphatidylethanolamine is the donor of the phosphorylethanolamine linked to the alpha1,4-linked mannose of yeast GPI structures. Glycobiology. 10, 1271-5.
- ↑ Kinoshita T. et al. (1995) Defective glycosyl-phosphatidylinositol anchor synthesis and paroxysmal nocturnal hemoglobinuria. Adv Immunol. 60, 57-103.
- ↑ Udenfriend S. and Kodukula K. (1995) How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. 64, 563-91.