Related Research Articles

A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

In metallurgy, a flux is a chemical cleaning agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining.

The gelatin silver process is the most commonly used chemical process in black-and-white photography, and is the fundamental chemical process for modern analog color photography. As such, films and printing papers available for analog photography rarely rely on any other chemical process to record an image. A suspension of silver salts in gelatin is coated onto a support such as glass, flexible plastic or film, baryta paper, or resin-coated paper. These light-sensitive materials are stable under normal keeping conditions and are able to be exposed and processed even many years after their manufacture. This was an improvement on the collodion wet-plate process dominant from the 1850s–1880s, which had to be exposed and developed immediately after coating.
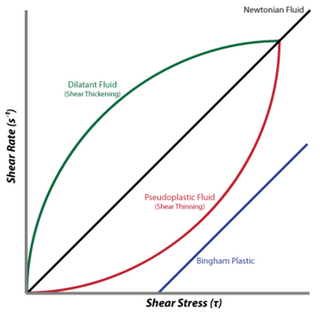
A dilatant material is one in which viscosity increases with the rate of shear strain. Such a shear thickening fluid, also known by the initialism STF, is an example of a non-Newtonian fluid. This behaviour is usually not observed in pure materials, but can occur in suspensions.
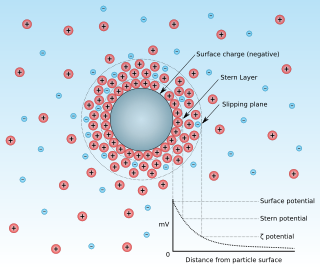
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.
The DLVO theory explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, . For two spheres of radius each having a charge separated by a center-to-center distance in a fluid of dielectric constant containing a concentration of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential,

Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion, in a liquid and are not truly dissolved in solution.

In chemistry, efflorescence is the migration of a salt to the surface of a porous material, where it forms a coating. The essential process involves the dissolving of an internally held salt in water, or occasionally in another solvent. The water, with the salt now held in solution, migrates to the surface, then evaporates, leaving a coating of the salt.
Surface charge is a two-dimensional surface with non-zero electric charge. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge distribution on the surface. The electric potential is continuous across a surface charge and the electric field is discontinuous, but not infinite; this is unless the surface charge consists of a dipole layer. In comparison, the potential and electric field both diverge at any point charge or linear charge.
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar or molal and to avoid confusion the units should be stated explicitly. The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes.
Electrocoagulation (EC) is a technique used for wastewater treatment, wash water treatment, industrially processed water, and medical treatment. Electrocoagulation has become a rapidly growing area of wastewater treatment due to its ability to remove contaminants that are generally more difficult to remove by filtration or chemical treatment systems, such as emulsified oil, total petroleum hydrocarbons, refractory organics, suspended solids, and heavy metals. There are many brands of electrocoagulation devices available and they can range in complexity from a simple anode and cathode to much more complex devices with control over electrode potentials, passivation, anode consumption, cell REDOX potentials as well as the introduction of ultrasonic sound, ultraviolet light and a range of gases and reactants to achieve so-called Advanced Oxidation Processes for refractory or recalcitrant organic substances.

Particle agglomeration refers to formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid phase stick to each other, and spontaneously form irregular particle assemblages, flocs, or agglomerates. This phenomenon is also referred to as coagulation or flocculation and such a suspension is also called unstable. Particle agglomeration can be induced by adding salts or other chemicals referred to as coagulant or flocculant.

Electroless nickel-phosphorus plating is a chemical process that deposits an even layer of nickel-phosphorus alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a phosphorus-containing reducing agent, usually a hypophosphite salt. It is the most common version of electroless nickel plating and is often referred by that name. A similar process uses a borohydride reducing agent, yielding a nickel-boron coating instead.
Photographic emulsion is a light-sensitive colloid used in film-based photography. Most commonly, in silver-gelatin photography, it consists of silver halide crystals dispersed in gelatin. The emulsion is usually coated onto a substrate of glass, films, paper, or fabric.
A streaming current and streaming potential are two interrelated electrokinetic phenomena studied in the areas of surface chemistry and electrochemistry. They are an electric current or potential which originates when an electrolyte is driven by a pressure gradient through a channel or porous plug with charged walls.
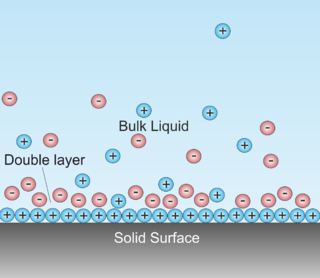
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". (-> this description of DL is not right, at least concerning the electrode/electrolyte interface. Here DL refers to charge separation at the interface with the electrode possessing negative charge and the electrolyte positive charge. The two layers are separated by some molecular distance. The two layers mentioned in above description are all at the electrolyte side.
Colloid-facilitated transport designates a transport process by which colloidal particles serve as transport vector of diverse contaminants in the surface water and in underground water circulating in fissured rocks (limestone, sandstone, granite, ...). The transport of colloidal particles in surface soils and in the ground can also occur, depending on the soil structure, soil compaction, and the particles size, but the importance of colloidal transport was only given sufficient attention during the 1980 years. Radionuclides, heavy metals, and organic pollutants, easily sorb onto colloids suspended in water and that can easily act as contaminant carrier.
Clarifying agents are used to remove suspended solids from liquids by inducing flocculation.

In water treatment, coagulation flocculation involves the addition of compounds that promote the clumping of fines into larger floc so that they can be more easily separated from the water. Coagulation is a chemical process that involves neutralization of charge whereas flocculation is a physical process and does not involve neutralization of charge. The coagulation-flocculation process can be used as a preliminary or intermediary step between other water or wastewater treatment processes like filtration and sedimentation. Iron and aluminium salts are the most widely used coagulants but salts of other metals such as titanium and zirconium have been found to be highly effective as well.
A protective colloid is a lyophilic colloid that when present in small quantities keeps lyophobic colloids from precipitating under the coagulating action of electrolytes.
References
- ↑ https://books.google.com/books?id=V3VIaBDTpJ8C Physical Pharmacy