Related Research Articles

In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can change into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.

Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. Metabolic composition, however, gets dramatically altered where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.
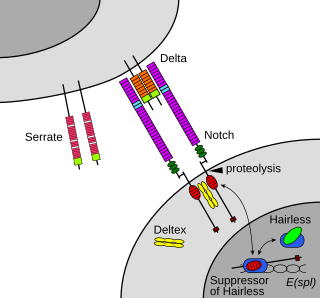
The Notch signaling pathway is a highly conserved cell signaling system present in most animals. Mammals possess four different notch receptors, referred to as NOTCH1, NOTCH2, NOTCH3, and NOTCH4. The notch receptor is a single-pass transmembrane receptor protein. It is a hetero-oligomer composed of a large extracellular portion, which associates in a calcium-dependent, non-covalent interaction with a smaller piece of the notch protein composed of a short extracellular region, a single transmembrane-pass, and a small intracellular region.

Embryoid bodies (EBs) are three-dimensional aggregates formed by pluripotent stem cells. These include embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC)

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.

A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced pluripotent stem cells. They are commonly used in research and regenerative medicine.
Hemangioblasts are the multipotent precursor cells that can differentiate into both hematopoietic and endothelial cells. In the mouse embryo, the emergence of blood islands in the yolk sac at embryonic day 7 marks the onset of hematopoiesis. From these blood islands, the hematopoietic cells and vasculature are formed shortly after. Hemangioblasts are the progenitors that form the blood islands. To date, the hemangioblast has been identified in human, mouse and zebrafish embryos.
Endothelial stem cells (ESCs) are one of three types of stem cells found in bone marrow. They are multipotent, which describes the ability to give rise to many cell types, whereas a pluripotent stem cell can give rise to all types. ESCs have the characteristic properties of a stem cell: self-renewal and differentiation. These parent stem cells, ESCs, give rise to progenitor cells, which are intermediate stem cells that lose potency. Progenitor stem cells are committed to differentiating along a particular cell developmental pathway. ESCs will eventually produce endothelial cells (ECs), which create the thin-walled endothelium that lines the inner surface of blood vessels and lymphatic vessels. The blood vessels include arteries and veins. Endothelial cells can be found throughout the whole vascular system and they also play a vital role in the movement of white blood cells

Paraxial mesoderm, also known as presomitic or somitic mesoderm, is the area of mesoderm in the neurulating embryo that flanks and forms simultaneously with the neural tube. The cells of this region give rise to somites, blocks of tissue running along both sides of the neural tube, which form muscle and the tissues of the back, including connective tissue and the dermis.

A mesoangioblast is a type of progenitor cell that is associated with vasculature walls. Mesoangioblasts exhibit many similarities to pericytes, which are found in the small vessels. Mesoangioblasts are multipotent stem cells with the potential to progress down the endothelial or mesodermal lineages. Mesoangioblasts express the critical marker of angiopoietic progenitors, KDR (FLK1). Because of these properties, mesoangioblasts are a precursor of skeletal, smooth, and cardiac muscle cells along with endothelial cells. Research has suggested their application for stem cell therapies for muscular dystrophy and cardiovascular disease.

Protein Wnt-3a is a protein that in humans is encoded by the WNT3A gene.
Stem cell markers are genes and their protein products used by scientists to isolate and identify stem cells. Stem cells can also be identified by functional assays. Below is a list of genes/protein products that can be used to identify various types of stem cells, or functional assays that do the same. The initial version of the list below was obtained by mining the PubMed database as described in

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.
P19 cells is an embryonic carcinoma cell line derived from an embryo-derived teratocarcinoma in mice. The cell line is pluripotent and can differentiate into cell types of all three germ layers. Also, it is the most characterized embryonic carcinoma (EC) cell line that can be induced into cardiac muscle cells and neuronal cells by different specific treatments. Indeed, exposing aggregated P19 cells to dimethyl sulfoxide (DMSO) induces differentiation into cardiac and skeletal muscle. Also, exposing P19 cells to retinoic acid (RA) can differentiate them into neuronal cells.
Endogenous cardiac stem cells (eCSCs) are tissue-specific stem progenitor cells harboured within the adult mammalian heart. A scientific-misconduct scandal, involving Harvard professor Piero Anversa, might indicate that the heart stem cell concept be broken. Therefore, the following article should be read with caution, as it builds on Anversa's results.

Fujifilm Cellular Dynamics, Inc. (FCDI) is a large scale manufacturer of human cells, created from induced pluripotent stem cells, for use in basic research, drug discovery and regenerative medicine applications.
Many human blood cells, such as red blood cells (RBCs), immune cells, and even platelets all originate from the same progenitor cell, the hematopoietic stem cell (HSC). As these cells are short-lived, there needs to be a steady turnover of new blood cells and the maintenance of an HSC pool. This is broadly termed hematopoiesis. This event requires a special environment, termed the hematopoietic stem cell niche, which provides the protection and signals necessary to carry out the differentiation of cells from HSC progenitors. This stem-cell niche relocates from the yolk sac to eventually rest in the bone marrow of mammals. Many pathological states can arise from disturbances in this niche environment, highlighting its importance in maintaining hematopoiesis.
Directed differentiation is a bioengineering methodology at the interface of stem cell biology, developmental biology and tissue engineering. It is essentially harnessing the potential of stem cells by constraining their differentiation in vitro toward a specific cell type or tissue of interest. Stem cells are by definition pluripotent, able to differentiate into several cell types such as neurons, cardiomyocytes, hepatocytes, etc. Efficient directed differentiation requires a detailed understanding of the lineage and cell fate decision, often provided by developmental biology.

Pancreatic progenitor cells are multipotent stem cells originating from the developing fore-gut endoderm which have the ability to differentiate into the lineage specific progenitors responsible for the developing pancreas.
References
- 1 2 3 4 Centre for Commercialization of Regenerative Medicine "Lead Scientist: Gordon Keller"
- ↑ Langelier, Julie (July 31, 2019). "The 2019 Ogawa-Yamanaka Stem Cell Prize Awarded to Gordon Keller". gladstone.org.
- ↑ University Health Network "Research Profile: Gordon Keller, PhD"
- ↑ "Behind the Breakthrough Podcast - University Health Network Season 4 - Dr. Gordon Keller" (PDF). Retrieved 14 December 2023.
- ↑ The Globe and Mail "Saskatchewan native Dr. Gordon Keller, a leader in regenerative medicine"
- ↑ University of Saskatchewan "Honorary Doctor of Science - Gordon Keller"
- ↑ Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G (1998). "A common precursor for hematopoietic and endothelial cells". Development. 125 (4): 725–732. doi:10.1242/dev.125.4.725. PMID 9435292.
- ↑ Han S, Dziedzic N, Gadue P, Keller GM, Gouon-Evans V (2011). "An endothelial cell niche induces hepatic specification through dual repression of Wnt and Notch signaling". Stem Cells. 29 (2): 217–228. doi:10.1002/stem.576. PMC 3437666 . PMID 21732480.
- ↑ Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, Daley GQ, Elefanty AG, Stanley EG, Keller G (2011). "Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells". Development. 138 (5): 861–871. doi:10.1242/dev.055236. PMC 3035090 . PMID 21270052.
- ↑ Kattman SJ, Huber TL, Keller GM (2006). "Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages". Developmental Cell. 11 (5): 723–732. doi: 10.1016/j.devcel.2006.10.002 . PMID 17084363.
- ↑ Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM (2013). "Specification of chondrocytes and cartilage tissues from embryonic stem cells". Development. 140 (12): 2597–2610. doi: 10.1242/dev.087890 . PMID 23715552.
- ↑ Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zúñiga-Pflücker JC, Keller G (2012). "T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures". Cell Reports. 2 (6): 1722–1735. doi: 10.1016/j.celrep.2012.11.003 . PMID 23219550.
- ↑ Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G (2014). "Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells". Nature Biotechnology. 32 (6): 554–561. doi:10.1038/nbt.2915. PMC 4152856 . PMID 24837661.
- ↑ "Development: Editorial Board".