Related Research Articles

Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis—or occasionally by more specialized processes of facilitated diffusion, passive transport or active transport. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of the egg. Note that a semipermeable membrane is not the same as a selectively permeable membrane. Semipermeable membrane describes a membrane that allows some particles to pass through, whereas the selectively permeable membrane will choose what passes through.
Ultrafiltration (UF) is a variety of membrane filtration in which forces like pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (103 - 106 Da) solutions, especially protein solutions.
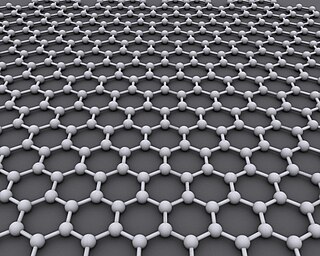
Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice. The name is a portmanteau of "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon consists of stacked graphene layers.
Thin-film composite membranes are semipermeable membranes manufactured principally for use in water purification or water desalination systems. They also have use in chemical applications such as batteries and fuel cells. A TFC membrane can be considered as a molecular sieve constructed in the form of a film from two or more layered materials.
Nanofiltration is a relatively recent membrane filtration process used most often with low total dissolved solids water such as surface water and fresh groundwater, with the purpose of softening and removal of disinfection by-product precursors such as natural organic matter and synthetic organic matter.

Sir Andre K. Geim is a Russian-born Dutch-British physicist working in England in the School of Physics and Astronomy at the University of Manchester.
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended chemical species as well as biological ones (principally bacteria) from water, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be "selective", this membrane should not allow large molecules or ions through the pores (holes), but should allow smaller components of the solution (such as solvent molecules, i.e., water, H2O) to pass freely.

Osmosis is the spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity.

Graphite oxide, formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers and acids for resolving of extra metals. The maximally oxidized bulk product is a yellow solid with C:O ratio between 2.1 and 2.9, that retains the layer structure of graphite but with a much larger and irregular spacing.
Cell theory has its origins in seventeenth century microscopy observations, but it was nearly two hundred years before a complete cell membrane theory was developed to explain what separates cells from the outside world. By the 19th century it was accepted that some form of semi-permeable barrier must exist around a cell. Studies of the action of anesthetic molecules led to the theory that this barrier might be made of some sort of fat (lipid), but the structure was still unknown. A series of pioneering experiments in 1925 indicated that this barrier membrane consisted of two molecular layers of lipids—a lipid bilayer. New tools over the next few decades confirmed this theory, but controversy remained regarding the role of proteins in the cell membrane. Eventually the fluid mosaic model was composed in which proteins “float” in a fluid lipid bilayer "sea". Although simplistic and incomplete, this model is still widely referenced today.
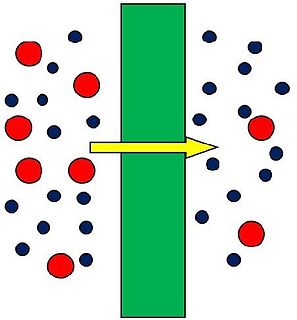
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Biological membranes include cell membranes ; nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry.
Carbocatalysis is a form of catalysis that uses heterogeneous carbon materials for the transformation or synthesis of organic or inorganic substrates. The catalysts are characterized by their high surface areas, surface functionality, and large, aromatic basal planes. Carbocatalysis can be distinguishable from supported catalysis in that no metal is present, or if metals are present they are not the active species.

Sir Konstantin Sergeevich Novoselov is a Russian-British physicist, and a Professor at the Centre for Advanced 2D Materials, National University of Singapore. He is also the Langworthy Professor in the School of Physics and Astronomy at the University of Manchester. His work on graphene with Andre Geim earned them the Nobel Prize in Physics in 2010.

Aerographite is a synthetic foam consisting of a porous interconnected network of tubular carbon. With a density of 180 g/m3 it is one of the lightest structural materials ever created. It was developed jointly by a team of researchers at the University of Kiel and the Technical University of Hamburg in Germany, and was first reported in a scientific journal in June 2012.
Potential graphene applications include lightweight, thin, and flexible electric/photonics circuits, solar cells, and various medical, chemical and industrial processes enhanced or enabled by the use of new graphene materials.
Hummers' method is a chemical process that can be used to generate graphite oxide through the addition of potassium permanganate to a solution of graphite, sodium nitrate, and sulfuric acid. It is commonly used by engineering and lab technicians as a reliable method of producing quantities of graphite oxide. It is also able to be revised in the creation of a one-molecule-thick version of the substance known as graphene oxide.
A rapidly increasing list of graphene production techniques have been developed to enable graphene's use in commercial applications.
Graphene is the only form of carbon in which every atom is available for chemical reaction from two sides. Atoms at the edges of a graphene sheet have special chemical reactivity. Graphene has the highest ratio of edge atoms of any allotrope. Defects within a sheet increase its chemical reactivity. The onset temperature of reaction between the basal plane of single-layer graphene and oxygen gas is below 260 °C (530 K). Graphene combusts at 350 °C (620 K). Graphene is commonly modified with oxygen- and nitrogen-containing functional groups and analyzed by infrared spectroscopy and X-ray photoelectron spectroscopy. However, determination of structures of graphene with oxygen- and nitrogen- functional groups requires the structures to be well controlled.

Single-layer graphene was explored theoretically by P. R. Wallace in 1947. It was first unambiguously produced and identified in 2004, by the group of Andre Geim and Konstantin Novoselov, though they credit Hanns-Peter Boehm and his co-workers for the experimental discovery of graphene in 1962. Boehm et al. introduced the term graphene in 1986.
There are many water purifiers available in the market which use different techniques like boiling, filtration, distillation, chlorination, sedimentation and oxidation. Currently nanotechnology plays a vital role in water purification techniques. Nanotechnology is the process of manipulating atoms on a nanoscale. In nanotechnology, nano membranes are used with the purpose of softening the water and removal of contaminants such as physical, biological and chemical contaminants. There are variety of techniques in nanotechnology which uses nano particles for providing safe drinking water with a high level of effectiveness. Some techniques have become commercialized.
References
- ↑ Jon Cartwright (2007-07-25). "Graphene oxide weaved into 'paper'". Physics World.
- ↑ "Graphene oxide paper could spawn a new class of materials". Phys.org. 2007-07-25.
- ↑ V. Kohlschütter; P. Haenni (1918). "Zur Kenntnis des Graphitischen Kohlenstoffs und der Graphitsäure". Z. Anorg. Allg. Chem. 105 (1): 121–144. doi:10.1002/zaac.19191050109.
- ↑ H. P. Boehm; A. Clauss; U. Hoffmann (1960). "Graphite oxide and its membrane properties". Journal de Chimie Physique. 58 (12): 110–117. Bibcode:1961JCP....58..141B. doi:10.1051/jcp/1961580141.
- ↑ R. R. Nair; H. A. Wu; P. N. Jayaram; I. V. Grigorieva; A. K. Geim (2012). "Unimpeded Permeation of Water Through Helium-Leak–Tight Graphene-Based Membranes". Science. 335 (6067): 442–444. arXiv: 1112.3488 . Bibcode:2012Sci...335..442N. doi:10.1126/science.1211694. PMID 22282806. S2CID 15204080.
- ↑ R. K. Joshi; P. Carbone; F. C. Wang; V. G. Kravets; Y. Su; I. V. Grigorieva; H. A. Wu; A. K. Geim; R. R. Nair (2014). "Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes". Science. 343 (6172): 752–754. arXiv: 1401.3134 . Bibcode:2014Sci...343..752J. doi:10.1126/science.1245711. PMID 24531966. S2CID 13154836.
- ↑ E. S. Bober (1970). Final report on reverse osmosis membranes containing graphitic oxide. U.S. Dept. of the Interior.
- Graphene Oxide Paper Could Spawn a New Class of Materials (Northwestern University Press Release)
- Graphene oxide weaved into 'paper' (physicsworld.com)
- It's Super Paper! (ScienceNOW Daily News)
- Ultrastrong Paper from Graphene (Technology Review)
- Carbon makes super-tough paper (Nature)