
Cholesterol is an organic molecule. It is a sterol, a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell membranes.
Juvenile hormones (JHs) are a group of acyclic sesquiterpenoids that regulate many aspects of insect physiology. The first discovery of a JH was by Vincent Wigglesworth. JHs regulate development, reproduction, diapause, and polyphenisms.

The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones.

Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth.

HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. Normally in mammalian cells this enzyme is suppressed by cholesterol derived from the internalization and degradation of low density lipoprotein (LDL) via the LDL receptor as well as oxidized species of cholesterol. Competitive inhibitors of the reductase induce the expression of LDL receptors in the liver, which in turn increases the catabolism of plasma LDL and lowers the plasma concentration of cholesterol, which is considered, by those who accept the standard lipid hypothesis, an important determinant of atherosclerosis. This enzyme is thus the target of the widely available cholesterol-lowering drugs known collectively as the statins.

Smith–Lemli–Opitz syndrome is an inborn error of cholesterol synthesis. It is an autosomal recessive, multiple malformation syndrome caused by a mutation in the enzyme 7-Dehydrocholesterol reductase encoded by the DHCR7 gene. It causes a broad spectrum of effects, ranging from mild intellectual disability and behavioural problems to lethal malformations.

Mevastatin is a hypolipidemic agent that belongs to the statins class.

Isopentenyl pyrophosphate is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
In enzymology, a Hydroxymethylglutaryl-CoA reductase (EC 1.1.1.88) is an enzyme that catalyzes the chemical reaction

β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl-CoA, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.
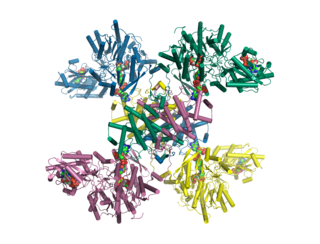
ATP citrate lyase (ACLY) is an enzyme that in animals represents an important step in fatty acid biosynthesis. By converting citrate to acetyl-CoA, the enzyme links carbohydrate metabolism, which yields citrate as an intermediate, with fatty acid biosynthesis, which consumes acetyl-CoA. In plants, ATP citrate lyase generates cytosolic acetyl-CoA precursors of thousands of specialized metabolites, including waxes, sterols, and polyketides.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

Lanosterol synthase is an oxidosqualene cyclase (OSC) enzyme that converts (S)-2,3-oxidosqualene to a protosterol cation and finally to lanosterol. Lanosterol is a key four-ringed intermediate in cholesterol biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene.

In enzymology, a hydroxymethylglutaryl-CoA reductase (NADPH) (EC 1.1.1.34) is an enzyme that catalyzes the chemical reaction
The discovery of HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase inhibitors, called statins, was a breakthrough in the prevention of hypercholesterolemia and related diseases. Hypercholesterolemia is considered to be one of the major risk factors for atherosclerosis which often leads to cardiovascular, cerebrovascular and peripheral vascular diseases. The statins inhibit cholesterol synthesis in the body and that leads to reduction in blood cholesterol levels, which is thought to reduce the risk of atherosclerosis and diseases caused by it.

Diphosphomevalonate decarboxylase (EC 4.1.1.33), most commonly referred to in scientific literature as mevalonate diphosphate decarboxylase, is an enzyme that catalyzes the chemical reaction

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.
In enzymology, a dephospho-[reductase kinase] kinase is an enzyme that catalyzes the chemical reaction

Insulin induced gene 1, also known as INSIG1, is a protein which in humans is encoded by the INSIG1 gene.
Statin-associated autoimmune myopathy (SAAM) is a very rare form of muscle damage caused by the immune system in people who take statin medications. However, there are cases of SAAM in patients who have not taken statin medication, and this can be explained by the exposure to natural sources of statin such as red yeast rice, which is statin rich. This theory is supported by the higher prevalence of statin-naive SAAM patients in Asian cohorts, who have statin-rich diets.















