Related Research Articles

Drosophila melanogaster is a species of fly in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly", or "banana fly". In the wild, D. melanogaster are attracted to rotting fruit and fermenting beverages, and are often found in orchards, kitchens and pubs.

Günter Blobel was a Silesian German and American biologist and 1999 Nobel Prize laureate in Physiology for the discovery that proteins have intrinsic signals that govern their transport and localization in the cell.

Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. The receptors TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antimicrobials which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.

Ralph Marvin Steinman was a Canadian physician and medical researcher at Rockefeller University, who in 1973 discovered and named dendritic cells while working as a postdoctoral fellow in the laboratory of Zanvil A. Cohn, also at Rockefeller University. Steinman was one of the recipients of the 2011 Nobel Prize in Physiology or Medicine.

Jules Alphonse Nicolas Hoffmann is a French biologist. During his youth, growing up in Luxembourg, he developed a strong interest in insects under the influence of his father, Jos Hoffmann. This eventually resulted in the younger Hoffmann's dedication to the field of biology using insects as model organisms. He currently holds a faculty position at the University of Strasbourg. He is a research director and member of the board of administrators of the National Center of Scientific Research (CNRS) in Strasbourg, France. He was elected to the positions of Vice-President (2005–2006) and President (2007–2008) of the French Academy of Sciences. Hoffmann and Bruce Beutler were jointly awarded a half share of the 2011 Nobel Prize in Physiology or Medicine for "their discoveries concerning the activation of innate immunity,". [More specifically, the work showing increased Drosomycin expression following activation of Toll pathway in microbial infection.]

Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro. However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies. It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence.
Priming is the first contact that antigen-specific T helper cell precursors have with an antigen. It is essential to the T helper cells' subsequent interaction with B cells to produce antibodies. Priming of antigen-specific naive lymphocytes occurs when antigen is presented to them in immunogenic form. Subsequently, the primed cells will differentiate either into effector cells or into memory cells that can mount stronger and faster response to second and upcoming immune challenges. T and B cell priming occurs in the secondary lymphoid organs.

Cecropins are antimicrobial peptides. They were first isolated from the hemolymph of Hyalophora cecropia, whence the term cecropin was derived. Cecropins lyse bacterial cell membranes; they also inhibit proline uptake and cause leaky membranes.
Richard L. Gallo is an American dermatologist who is a Distinguished Professor and founding Chairman of Dermatology at the University of California, San Diego. His research accomplishments as a physician-scientist include discovery of antimicrobial peptides in mammalian skin, establishing new links between innate immunity and skin diseases such as atopic dermatitis and rosacea, and defining the functions of the skin microbiome in host immune defense.
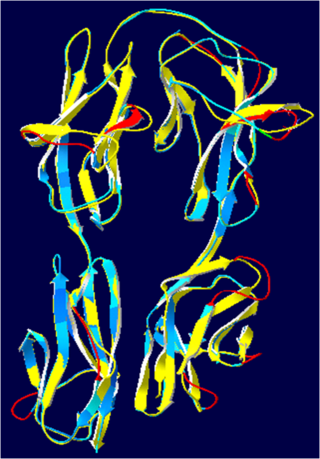
Hemolin is an immunoglobulin-like protein exclusively found in Lepidoptera. It was first discovered in immune-challenged pupae of Hyalophora cecropia and Manduca sexta.

Attacin is a glycine-rich protein of about 20 kDa belonging to the group of antimicrobial peptides (AMP). It is active against Gram-negative bacteria.

Drosomycin is an antifungal peptide from Drosophila melanogaster and was the first antifungal peptide isolated from insects. Drosomycin is induced by infection by the Toll signalling pathway, while expression in surface epithelia like the respiratory tract is instead controlled by the immune deficiency pathway (Imd). This means that drosomycin, alongside other antimicrobial peptides (AMPs) such as cecropins, diptericin, drosocin, metchnikowin and attacin, serves as a first line defence upon septic injury. However drosomycin is also expressed constitutively to a lesser extent in different tissues and throughout development.

Dan Hultmark is a Swedish biologist currently Professor Emeritus, whose research focused on the mechanisms of innate immunity, using Drosophila as a model system, at Umeå University and an Elected Fellow of the American Association for the Advancement of Science. Hultmark is also a member of the Drosophila 12 Genomes Consortium, Tribolium Genome Sequencing Consortium.

Robert Ernest William Hancock is a Canadian microbiologist and University of British Columbia Killam Professor of Microbiology and Immunology, an Associate Faculty Member of the Wellcome Trust Sanger Institute, and a Canada Research Chair in Health and Genomics.

Diptericin is a 9 kDa antimicrobial peptide (AMP) of flies first isolated from the blowfly Phormia terranova. It is primarily active against Gram-negative bacteria, disrupting bacterial membrane integrity. The structure of this protein includes a proline-rich domain with similarities to the AMPs drosocin, pyrrhocoricin, and abaecin, and a glycine-rich domain with similarity to attacin. Diptericin is an iconic readout of immune system activity in flies, used ubiquitously in studies of Drosophila immunity. Diptericin is named after the insect order Diptera.
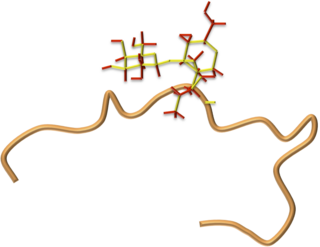
Drosocin is a 19-residue long antimicrobial peptide (AMP) of flies first isolated in the fruit fly Drosophila melanogaster, and later shown to be conserved throughout the genus Drosophila. Drosocin is regulated by the NF-κB Imd signalling pathway in the fly.

The Imd pathway is a broadly-conserved NF-κB immune signalling pathway of insects and some arthropods that regulates a potent antibacterial defence response. The pathway is named after the discovery of a mutation causing severe immune deficiency. The Imd pathway was first discovered in 1995 using Drosophila fruit flies by Bruno Lemaitre and colleagues, who also later discovered that the Drosophila Toll gene regulated defence against Gram-positive bacteria and fungi. Together the Toll and Imd pathways have formed a paradigm of insect immune signalling; as of September 2, 2019, these two landmark discovery papers have been cited collectively over 5000 times since publication on Google Scholar.

Bruno Lemaitre is a French immunologist and a professor at the École Polytechnique Fédérale de Lausanne (EPFL). His research focuses on the mechanisms of innate immunity and endosymbiosis in Drosophila. Lemaitre has also authored several books on the topic of narcissism in science. and a book on the philosophy of Michael Polanyi.

The Bomanin gene family encodes a group of immune peptides that are essential for Drosophila fruit fly defence against infection by many pathogens.
References
- ↑ Umeå professor linked to Nobel Prize winning research. https://www.umu.se/en/news/umea-professor-linked-to-nobel-prize-winning-research_5832655/
- ↑ Pütsep, Katrin; Carlsson, Göran; Boman, Hans G.; Andersson, Mats (2002). "Deficiency of antibacterial peptides in patients with morbus Kostmann: An observation study". The Lancet. 360 (9340): 1144–1149. doi:10.1016/S0140-6736(02)11201-3. PMID 12387964. S2CID 40904911.
- ↑ Faye, Ingrid; Lindberg, Bo G. (2016). "Towards a paradigm shift in innate immunity—seminal work by Hans G. Boman and co-workers". Philosophical Transactions of the Royal Society B: Biological Sciences. 371 (1695): 20150303. doi:10.1098/rstb.2015.0303. PMC 4874399 . PMID 27160604.
- ↑ Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H. G. (1981). "Sequence and specificity of two antibacterial proteins involved in insect immunity". Nature. 292 (5820): 246–248. Bibcode:1981Natur.292..246S. doi:10.1038/292246a0. PMID 7019715. S2CID 4269791.
- ↑ "The Nobel Prize in Physiology or Medicine 2011".
- ↑ Pütsep, K.; Faye, I. (2009). "Hans G Boman (1924-2008): Pioneer in Peptide-Mediated Innate Immune Defence". Scandinavian Journal of Immunology. 70 (3): 317–319. doi:10.1111/j.1365-3083.2009.02293.x. PMID 19703022.
- ↑ "Minisymposium to honour Hans G. Boman - Department of Molecular Biosciences, the Wenner-Gren Institute".
- ↑ Clemmons, Alexa W.; Lindsay, Scott A.; Wasserman, Steven A. (2015). "An Effector Peptide Family Required for Drosophila Toll-Mediated Immunity". PLOS Pathogens. 11 (4): e1004876. doi: 10.1371/journal.ppat.1004876 . PMC 4411088 . PMID 25915418.