
In physics, cryogenics is the production and behaviour of materials at very low temperatures.

Refrigeration is any of various types of cooling of a space, substance, or system to lower and/or maintain its temperature below the ambient one. Refrigeration is an artificial, or human-made, cooling method.
The following is a timeline of low-temperature technology and cryogenic technology. It also lists important milestones in thermometry, thermodynamics, statistical physics and calorimetry, that were crucial in development of low temperature systems.
A cryopump or a "cryogenic pump" is a vacuum pump that traps gases and vapours by condensing them on a cold surface, but are only effective on some gases. The effectiveness depends on the freezing and boiling points of the gas relative to the cryopump's temperature. They are sometimes used to block particular contaminants, for example in front of a diffusion pump to trap backstreaming oil, or in front of a McLeod gauge to keep out water. In this function, they are called a cryotrap, waterpump or cold trap, even though the physical mechanism is the same as for a cryopump.

Liquid helium is a physical state of helium at very low temperatures at standard atmospheric pressures. Liquid helium may show superfluidity.

A 3He/4He dilution refrigerator is a cryogenic device that provides continuous cooling to temperatures as low as 2 mK, with no moving parts in the low-temperature region. The cooling power is provided by the heat of mixing of the helium-3 and helium-4 isotopes.

An evaporative cooler is a device that cools air through the evaporation of water. Evaporative cooling differs from other air conditioning systems, which use vapor-compression or absorption refrigeration cycles. Evaporative cooling exploits the fact that water will absorb a relatively large amount of heat in order to evaporate. The temperature of dry air can be dropped significantly through the phase transition of liquid water to water vapor (evaporation). This can cool air using much less energy than refrigeration. In extremely dry climates, evaporative cooling of air has the added benefit of conditioning the air with more moisture for the comfort of building occupants.
A refrigerator designed to reach cryogenic temperatures is often called a cryocooler. The term is most often used for smaller systems, typically table-top size, with input powers less than about 20 kW. Some can have input powers as low as 2–3 W. Large systems, such as those used for cooling the superconducting magnets in particle accelerators are more often called cryogenic refrigerators. Their input powers can be as high as 1 MW. In most cases cryocoolers use a cryogenic fluid as the working substance and employ moving parts to cycle the fluid around a thermodynamic cycle. The fluid is typically compressed at room temperature, precooled in a heat exchanger, then expanded at some low temperature. The returning low-pressure fluid passes through the heat exchanger to precool the high-pressure fluid before entering the compressor intake. The cycle is then repeated.
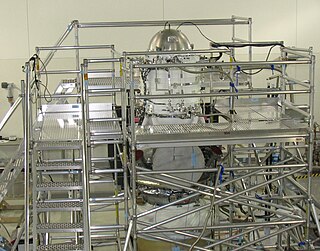
A cryostat is a device used to maintain low cryogenic temperatures of samples or devices mounted within the cryostat. Low temperatures may be maintained within a cryostat by using various refrigeration methods, most commonly using cryogenic fluid bath such as liquid helium. Hence it is usually assembled into a vessel, similar in construction to a vacuum flask or Dewar. Cryostats have numerous applications within science, engineering, and medicine.

A refrigerator, commonly fridge, is a commercial and home appliance consisting of a thermally insulated compartment and a heat pump that transfers heat from its inside to its external environment so that its inside is cooled to a temperature below the room temperature. Refrigeration is an essential food storage technique around the world. The low temperature reduces the reproduction rate of bacteria, so the refrigerator lowers the rate of spoilage. A refrigerator maintains a temperature a few degrees above the freezing point of water. The optimal temperature range for perishable food storage is 3 to 5 °C. A freezer is a specialized refrigerator, or portion of a refrigerator, that maintains its contents’ temperature below the freezing point of water. The refrigerator replaced the icebox, which had been a common household appliance for almost a century and a half. The United States Food and Drug Administration recommends that the refrigerator be kept at or below 4 °C (40 °F) and that the freezer be regulated at −18 °C (0 °F).
A 1-K pot is a cryogenic device used to attain temperatures down to approximately 1 kelvin.

The Coolgardie safe is a low-tech food storage unit, using evaporative cooling to prolong the life of whatever edibles are kept in it. It applies the basic principle of heat transfer which occurs during evaporation of water. It was named after the place where it was invented – the small mining town of Coolgardie, Western Australia, near Kalgoorlie-Boulder.
Auto-defrost, automatic defrost or self-defrosting is a technique which regularly defrosts the evaporator in a refrigerator or freezer. Appliances using this technique are often called frost free, frostless, or no-frost.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. Solar energy, burning a fossil fuel, waste heat from factories, and district heating systems are examples of convenient heat sources that can be used. An absorption refrigerator uses two coolants: the first coolant performs evaporative cooling and then is absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Absorption refrigerators can also be used to air-condition buildings using the waste heat from a gas turbine or water heater in the building. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration.

A pot-in-pot refrigerator, clay pot cooler or zeer is an evaporative cooling refrigeration device which does not use electricity. It uses a porous outer clay pot containing an inner pot within which the food is placed. The evaporation of the outer liquid draws heat from the inner pot. The device can cool any substance, and requires only a flow of relatively dry air and a source of water.

The solar transition region is a region of the Sun's atmosphere between the upper chromosphere and corona. It is important because it is the site of several unrelated but important transitions in the physics of the solar atmosphere:

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.
Mohammed Bah Abba (1964–2010) was a teacher from northern Nigeria who developed the pot-in-pot refrigerator in the 1990s. This refrigerator is extremely simple and does not require power, making it suitable for use in desert environments without easy access to electricity or repairs. It consists of a small glazed earthenware pot placed inside a larger unglazed one, with the space between the two filled with moist sand. The inner pot is filled with whatever is desired to be cooled and covered with a wet cloth. The evaporating water draws heat from the inside through the porous outer pot, cooling the interior by up to 14°C.
A lambda point refrigerator is a device used to cool liquid helium, typically around a superconducting magnet or for low temperature measurements, from approximately 4.2 K to temperatures near the lambda point of helium, the temperature at which normal fluid helium transitions to the superfluid helium II. Cooling is achieved by pumping the liquid helium in the bath through a cooling coil via a needle valve and vacuum pump. The reduced pressure in the coil causes some of the helium to evaporate, creating a two-phase system within the cooling coil. The heat removed via evaporation lowers the temperature of the cooling coil closer to the lambda point. Since the cooling coil is immersed in the liquid helium bath, liquid surrounding the coil is also cooled. The colder, higher density liquid sinks away from the coil toward the bottom of the bath while the warmer, lower density liquid helium rises to the top. Liquid helium typically has poor thermal conductivity, so convective currents associated with a temperature gradient in the bath provide a constant flow of this colder liquid helium toward the bottom of the bath, allowing temperatures below 4.2 K to be realized in the helium bath, typically close to 2.2 K.

The pulse tube refrigerator (PTR) or pulse tube cryocooler is a developing technology that emerged largely in the early 1980s with a series of other innovations in the broader field of thermoacoustics. In contrast with other cryocoolers, this cryocooler can be made without moving parts in the low temperature part of the device, making the cooler suitable for a wide variety of applications.











