
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
Superfluid helium-4 is the superfluid form of helium-4, an isotope of the element helium. A superfluid is a state of matter in which matter behaves like a fluid with zero viscosity. The substance, which resembles other liquids such as helium I, flows without friction past any surface, which allows it to continue to circulate over obstructions and through pores in containers which hold it, subject only to its own inertia.
The following is a timeline of low-temperature technology and cryogenic technology. It also lists important milestones in thermometry, thermodynamics, statistical physics and calorimetry, that were crucial in development of low temperature systems.
A cryopump or a "cryogenic pump" is a vacuum pump that traps gases and vapours by condensing them on a cold surface, but are only effective on some gases. The effectiveness depends on the freezing and boiling points of the gas relative to the cryopump's temperature. They are sometimes used to block particular contaminants, for example in front of a diffusion pump to trap backstreaming oil, or in front of a McLeod gauge to keep out water. In this function, they are called a cryotrap, waterpump or cold trap, even though the physical mechanism is the same as for a cryopump.

Magnetic refrigeration is a cooling technology based on the magnetocaloric effect. This technique can be used to attain extremely low temperatures, as well as the ranges used in common refrigerators.

Liquid helium is a physical state of helium at very low temperatures at standard atmospheric pressures. Liquid helium may show superfluidity.

A superconducting magnet is an electromagnet made from coils of superconducting wire. They must be cooled to cryogenic temperatures during operation. In its superconducting state the wire has no electrical resistance and therefore can conduct much larger electric currents than ordinary wire, creating intense magnetic fields. Superconducting magnets can produce stronger magnetic fields than all but the strongest non-superconducting electromagnets, and large superconducting magnets can be cheaper to operate because no energy is dissipated as heat in the windings. They are used in MRI instruments in hospitals, and in scientific equipment such as NMR spectrometers, mass spectrometers, fusion reactors and particle accelerators. They are also used for levitation, guidance and propulsion in a magnetic levitation (maglev) railway system being constructed in Japan.
Thermoacoustics is the interaction between temperature, density and pressure variations of acoustic waves. Thermoacoustic heat engines can readily be driven using solar energy or waste heat and they can be controlled using proportional control. They can use heat available at low temperatures which makes it ideal for heat recovery and low power applications. The components included in thermoacoustic engines are usually very simple compared to conventional engines. The device can easily be controlled and maintained.
A refrigerator designed to reach cryogenic temperatures is often called a cryocooler. The term is most often used for smaller systems, typically table-top size, with input powers less than about 20 kW. Some can have input powers as low as 2–3 W. Large systems, such as those used for cooling the superconducting magnets in particle accelerators are more often called cryogenic refrigerators. Their input powers can be as high as 1 MW. In most cases cryocoolers use a cryogenic fluid as the working substance and employ moving parts to cycle the fluid around a thermodynamic cycle. The fluid is typically compressed at room temperature, precooled in a heat exchanger, then expanded at some low temperature. The returning low-pressure fluid passes through the heat exchanger to precool the high-pressure fluid before entering the compressor intake. The cycle is then repeated.
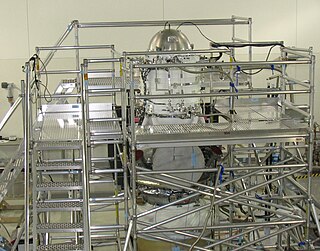
A cryostat is a device used to maintain low cryogenic temperatures of samples or devices mounted within the cryostat. Low temperatures may be maintained within a cryostat by using various refrigeration methods, most commonly using cryogenic fluid bath such as liquid helium. Hence it is usually assembled into a vessel, similar in construction to a vacuum flask or Dewar. Cryostats have numerous applications within science, engineering, and medicine.
A 1-K pot is a cryogenic device used to attain temperatures down to approximately 1 kelvin.
Helium (2He) has nine known isotopes, but only helium-3 (3He) and helium-4 (4He) are stable. All radioisotopes are short-lived; the longest-lived is 6He with half-life 806.92(24) milliseconds. The least stable is 10He, with half-life 260(40) yoctoseconds, though 2He may have an even shorter half-life.

Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and expansions to achieve high pressures and very low temperatures, using, for example, turboexpanders.
Heliox is a cryogenically cooled system produced by Oxford Instruments.

The pulse tube refrigerator (PTR) or pulse tube cryocooler is a developing technology that emerged largely in the early 1980s with a series of other innovations in the broader field of thermoacoustics. In contrast with other cryocoolers, this cryocooler can be made without moving parts in the low temperature part of the device, making the cooler suitable for a wide variety of applications.

TheVuilleumier cycle was patented by a Swiss-American engineer named Rudolph Vuilleumier in 1918. The purpose of Vuilleumier's machine was to create a heat pump that would use heat at high temperature as energy input. The Vuilleumier cycle...
utilize[s] working gas expansion and compression at three variable volume spaces in order to pump heat from a low to a moderate temperature level. The interesting characteristic of the Vuilleumier machine is that the induced volume variations are realized without the use of work, but thermally. This is the reason why it has a potential to operate at modern applications where the pollution of the environment is not a choice. It is a perfect candidate for such applications, as it consists only of metallic parts and inert gas. Using these units for heating and cooling buildings, large energy savings can be accomplished as they can be operated at small scale in common buildings or at large scale providing heat power to entire building blocks without using fossil fuels. The use of Vuilleumier machines for industrial applications or inside vehicles is also a feasible option. Another field where these machines have already been involved is cryogenics, as they are also able to provide refrigeration at very low temperatures like the very similar and well-known Stirling refrigerators.

Stefan Janos is a Slovak-Swiss university physicist and professor, founder of very low temperature physics in Slovakia.
Howard Oldford McMahon (1914–1990) was an American electrical engineer who was Science Director, Vice President, Head of the Research and Development Division, and then President of Arthur D. Little, Inc, of Cambridge, Massachusetts, retiring from the Company in 1977. He was born in Alberta, Canada, and became a naturalized citizen of the United States. He made contributions to the field of cryogenics as inventor during the 1940s through the 1960s, and subsequently as an executive and member of the Board of Directors of both ADL and the Helix Technology Corporation, of Waltham, Massachusetts.
The polarized targets are used as fixed targets in scattering experiments. In high energy physics they are used to study the nucleon spin structure of simple nucleons like protons, neutrons or deuterons. In deep inelastic scattering the hadron structure is probed with electrons, muons or neutrinos. Using a polarized high energy muon beam, for example, on a fixed target with polarized nucleons it is possible to probe the spin dependent part of the structure functions.

In the field of cryogenics, helium [He] is utilized for a variety of reasons. The combination of helium’s extremely low molecular weight and weak interatomic reactions yield interesting properties when helium is cooled below its critical temperature of 5.2 K to form a liquid. Even at absolute zero (0K), helium does not condense to form a solid under ambient pressure. In this state, the zero point vibrational energies of helium are comparable to very weak interatomic binding interactions, thus preventing lattice formation and giving helium its fluid characteristics. Within this liquid state, helium has two phases referred to as helium I and helium II. Helium I displays thermodynamic and hydrodynamic properties of classical fluids, along with quantum characteristics. However, below its lambda point of 2.17 K, helium transitions to He II and becomes a quantum superfluid with zero viscosity.



















