
A heat engine is a system that converts heat to usable energy, particularly mechanical energy, which can then be used to do mechanical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag.

Centrifugal compressors, sometimes called impeller compressors or radial compressors, are a sub-class of dynamic axisymmetric work-absorbing turbomachinery.

The Brayton cycle, also known as the Joule cycle, is a thermodynamic cycle that describes the operation of certain heat engines that have air or some other gas as their working fluid. It is characterized by isentropic compression and expansion, and isobaric heat addition and rejection, though practical engines have adiabatic rather than isentropic steps.

A compressor is a mechanical device that increases the pressure of a gas by reducing its volume. An air compressor is a specific type of gas compressor.

The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, adsorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.
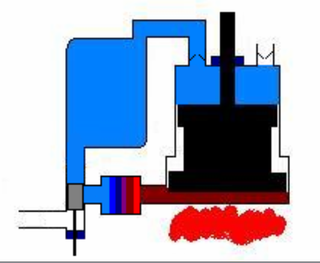
The Ericsson cycle is named after inventor John Ericsson who designed and built many unique heat engines based on various thermodynamic cycles. He is credited with inventing two unique heat engine cycles and developing practical engines based on these cycles. His first cycle is now known as the closed Brayton cycle, while his second cycle is what is now called the Ericsson cycle. Ericsson is one of the few who built open-cycle engines, but he also built closed-cycle ones.
An air cycle machine (ACM) is the refrigeration unit of the environmental control system (ECS) used in pressurized gas turbine-powered aircraft. Normally an aircraft has two or three of these ACM. Each ACM and its components are often referred as an air conditioning pack. The air cycle cooling process uses air instead of a phase changing material such as Freon in the gas cycle. No condensation or evaporation of a refrigerant is involved, and the cooled air output from the process is used directly for cabin ventilation or for cooling electronic equipment.

A thermodynamic cycle consists of linked sequences of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. If at every point in the cycle the system is in thermodynamic equilibrium, the cycle is reversible. Whether carried out reversible or irreversibly, the net entropy change of the system is zero, as entropy is a state function.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. Solar energy, burning a fossil fuel, waste heat from factories, and district heating systems are examples of heat sources that can be used. An absorption refrigerator uses two coolants: the first coolant performs evaporative cooling and then is absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Absorption refrigerators can also be used to air-condition buildings using the waste heat from a gas turbine or water heater in the building. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration.

In thermodynamics, the thermal efficiency is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
Economizers, or economisers (UK), are mechanical devices intended to reduce energy consumption, or to perform useful function such as preheating a fluid. The term economizer is used for other purposes as well. Boiler, power plant, heating, refrigeration, ventilating, and air conditioning (HVAC) may all use economizers. In simple terms, an economizer is a heat exchanger.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.

A turboexpander, also referred to as a turbo-expander or an expansion turbine, is a centrifugal or axial-flow turbine, through which a high-pressure gas is expanded to produce work that is often used to drive a compressor or generator.

A thermal expansion valve or thermostatic expansion valve is a component in vapor-compression refrigeration and air conditioning systems that controls the amount of refrigerant released into the evaporator and is intended to regulate the superheat of the refrigerant that flows out of the evaporator to a steady value. Although often described as a "thermostatic" valve, an expansion valve is not able to regulate the evaporator's temperature to a precise value. The evaporator's temperature will vary only with the evaporating pressure, which will have to be regulated through other means.

A transcritical cycle is a closed thermodynamic cycle where the working fluid goes through both subcritical and supercritical states. In particular, for power cycles the working fluid is kept in the liquid region during the compression phase and in vapour and/or supercritical conditions during the expansion phase. The ultrasupercritical steam Rankine cycle represents a widespread transcritical cycle in the electricity generation field from fossil fuels, where water is used as working fluid. Other typical applications of transcritical cycles to the purpose of power generation are represented by organic Rankine cycles, which are especially suitable to exploit low temperature heat sources, such as geothermal energy, heat recovery applications or waste to energy plants. With respect to subcritical cycles, the transcritical cycle exploits by definition higher pressure ratios, a feature that ultimately yields higher efficiencies for the majority of the working fluids. Considering then also supercritical cycles as a valid alternative to the transcritical ones, the latter cycles are capable of achieving higher specific works due to the limited relative importance of the work of compression work. This evidences the extreme potential of transcritical cycles to the purpose of producing the most power with the least expenditure.

Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that transmits heat from one location at a certain temperature to another location at a higher temperature. Thus a heat pump may be thought of as a "heater" if the objective is to warm the heat sink, or a "refrigerator" or “cooler” if the objective is to cool the heat source. The operating principles in both cases are the same; energy is used to move heat from a colder place to a warmer place.
The term subcooling refers to the intentional process of cooling a liquid below its normal boiling point. For example, water boils at 373 K; at room temperature (293 K) liquid water is termed "subcooled". Subcooling is a common stage in refrigeration cycles and steam turbine cycles. Some rocket engines use subcooled propellants.
In refrigeration, flash-gas is refrigerant in gas form produced spontaneously when the condensed liquid is subjected to boiling. The presence of flash-gas in the liquid lines reduces the efficiency of the refrigeration cycle. It can also lead several expansion systems to work improperly, and increase superheating at the evaporator. This is normally perceived as an unwanted condition caused by dissociation between the volume of the system, and the pressures and temperatures that allow the refrigerant to be liquid. Flash-gas must not be confused with lack of condensation, but special gear such as receivers, internal heat exchangers, insulation, and refrigeration cycle optimizers may improve condensation and avoid gas in the liquid lines.
Heat engines, refrigeration cycles and heat pumps usually involve a fluid to and from which heat is transferred while undergoing a thermodynamic cycle. This fluid is called the working fluid. Refrigeration and heat pump technologies often refer to working fluids as refrigerants. Most thermodynamic cycles make use of the latent heat of the working fluid. In case of other cycles the working fluid remains in gaseous phase while undergoing all the processes of the cycle. When it comes to heat engines, working fluid generally undergoes a combustion process as well, for example in internal combustion engines or gas turbines. There are also technologies in heat pump and refrigeration, where working fluid does not change phase, such as reverse Brayton or Stirling cycle.














