Related Research Articles

In chemistry, phosphorylation of a molecule is the attachment of a phosphoryl group. This process and its inverse, dephosphorylation, are critical for many cellular processes in biology. Protein phosphorylation is especially important for their function; for example, this modification activates almost half of the enzymes present in Saccharomyces cerevisiae, thereby regulating their function. Many proteins are phosphorylated temporarily, as are many sugars, lipids, and other biologically-relevant molecules.
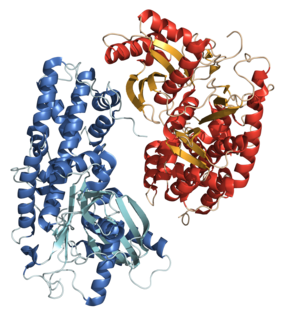
A hexokinase is an enzyme that phosphorylates hexoses, forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate.

Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.

The lactose operon is an operon required for the transport and metabolism of lactose in E.coli and many other enteric bacteria. Although glucose is the preferred carbon source for most bacteria, the lac operon allows for the effective digestion of lactose when glucose is not available through the activity of beta-galactosidase. Gene regulation of the lac operon was the first genetic regulatory mechanism to be understood clearly, so it has become a foremost example of prokaryotic gene regulation. It is often discussed in introductory molecular and cellular biology classes for this reason. This lactose metabolism system was used by François Jacob and Jacques Monod to determine how a biological cell knows which enzyme to synthesize. Their work on the lac operon won them the Nobel Prize in Physiology in 1965.
PEP group translocation, also known as the phosphotransferase system or PTS, is a distinct method used by bacteria for sugar uptake where the source of energy is from phosphoenolpyruvate (PEP). It is known to be a multicomponent system that always involves enzymes of the plasma membrane and those in the cytoplasm.
The galactose permease or GalP found in Escherichia coli is an integral membrane protein involved in the transport of monosaccharides, primarily hexoses, for utilization by E. coli in glycolysis and other metabolic and catabolic pathways (3,4). It is a member of the Major Facilitator Super Family (MFS) and is homologue of the human GLUT1 transporter (4). Below you will find descriptions of the structure, specificity, effects on homeostasis, expression, and regulation of GalP along with examples of several of its homologues.

Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.

SgrS is a 227 nucleotide small RNA that is activated by SgrR in Escherichia coli during glucose-phosphate stress. The nature of glucose-phosphate stress is not fully understood, but is correlated with intracellular accumulation of glucose-6-phosphate. SgrS helps cells recover from glucose-phosphate stress by base pairing with ptsG mRNA and causing its degradation in an RNase E dependent manner. Base pairing between SgrS and ptsG mRNA also requires Hfq, an RNA chaperone frequently required by small RNAs that affect their targets through base pairing. The inability of cells expressing sgrS to create new glucose transporters leads to less glucose uptake and reduced levels of glucose-6-phosphate. SgrS is an unusual small RNA in that it also encodes a 43 amino acid functional polypeptide, SgrT, which helps cells recover from glucose-phosphate stress by preventing glucose uptake. The activity of SgrT does not affect the levels of ptsG mRNA of PtsG protein. It has been proposed that SgrT exerts its effects through regulation of the glucose transporter, PtsG.

Outer membrane receptors, also known as TonB-dependent receptors, are a family of beta barrel proteins named for their localization in the outer membrane of gram-negative bacteria. TonB complexes sense signals from the outside of bacterial cells and transmit them into the cytoplasm, leading to transcriptional activation of target genes. TonB-dependent receptors in gram-negative bacteria are associated with the uptake and transport of large substrates such as iron siderophore complexes and vitamin B12.
The gal operon is a prokaryotic operon, which encodes enzymes necessary for galactose metabolism. Repression of gene expression for this operon works via binding of repressor molecules to two operators. These repressors dimerize, creating a loop in the DNA. The loop as well as hindrance from the external operator prevent RNA polymerase from binding to the promoter, and thus prevent transcription. Additionally, since the metabolism of galactose in the cell is involved in both anabolic and catabolic pathways, a novel regulatory system using two promoters for differential repression has been identified and characterized within the context of the gal operon.

In molecular biology, the LuxR-type DNA-binding HTH domain is a DNA-binding, helix-turn-helix (HTH) domain of about 65 amino acids. It is present in transcription regulators of the LuxR/FixJ family of response regulators. The domain is named after Vibrio fischeri luxR, a transcriptional activator for quorum-sensing control of luminescence. LuxR-type HTH domain proteins occur in a variety of organisms. The DNA-binding HTH domain is usually located in the C-terminal region of the protein; the N-terminal region often containing an autoinducer-binding domain or a response regulatory domain. Most luxR-type regulators act as transcription activators, but some can be repressors or have a dual role for different sites. LuxR-type HTH regulators control a wide variety of activities in various biological processes.
EnvZ/OmpR is a two-component regulatory system widely distributed in bacteria and particularly well characterized in Escherichia coli. Its function is in osmoregulation, responding to changes in environmental osmolality by regulating the expression of the outer membrane porins OmpF and OmpC. EnvZ is a histidine kinase which also possesses a cytoplasmic osmosensory domain, and OmpR is its corresponding response regulator protein.

A response regulator is a protein that mediates a cell's response to changes in its environment as part of a two-component regulatory system. Response regulators are coupled to specific histidine kinases which serve as sensors of environmental changes. Response regulators and histidine kinases are two of the most common gene families in bacteria, where two-component signaling systems are very common; they also appear much more rarely in the genomes of some archaea, yeasts, filamentous fungi, and plants. Two-component systems are not found in metazoans.
The glnALG operon is an operon that regulates the nitrogen content of a cell. It codes for the structural gene glnA the two regulatory genes glnL and glnG. glnA encodes glutamine synthetase, an enzyme which catalyzes the conversion of glutamate and ammonia to glutamine, thereby controlling the nitrogen level in the cell. glnG encodes NRI which regulates the expression of the glnALG operon at three promoters, which are glnAp1, glnAp2 located upstream of glnA) and glnLp. glnL encodes NRII which regulates the activity of NRI. No significant homology is found in Eukaryotes.
In bacterial genetics, the mal regulon is a regulon - or group of genes under common regulation - associated with the catabolism of maltose and maltodextrins. The system is especially well characterized in the model organism Escherichia coli, where it is classically described as a group of ten genes in multiple operons whose expression is regulated by a single regulatory protein, malT. MalT binds to maltose or maltodextrin and undergoes a conformational change that allows it to bind DNA at sequences near the promoters of genes required for uptake and catabolism of these sugars. The maltose regulation system in E. coli is a classic example of positive regulation. malT is regulated by catabolite repression via the catabolite activator protein. Genes under the control of malT include ATP-binding cassette transporter components, maltoporin, maltose binding protein, and several enzymes. Other Gram-negative bacteria such as Klebsiella pneumoniae have additional genes under the control of malT.

ZnuABC is a high-affinity transporter specialized for transporting zinc ions as part of a system for metal ion homeostasis in bacteria. The complex is a member of the ATP-binding cassette (ABC) transporter protein family. The transporter contains three protein components:
The PTS L-Ascorbate (L-Asc) Family includes porters specific for L-ascorbate, and is part of the PTS-AG superfamily. A single PTS permease of the L-Asc family of PTS permeases has been functionally characterized. This is the SgaTBA system, renamed UlaABC by Yew and Gerlt.
The PTS Mannose-Fructose-Sorbose (Man) Family is a group of multicomponent PTS systems that are involved in sugar uptake in bacteria. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB) as well as the integral membrane sugar permease complex (IICD). It is not part of the PTS-AG or PTS-GFL superfamilies.

The Phosphate (Pho) regulon is a bacterial regulatory mechanism used for the conservation and management of inorganic phosphate within the cell. It was first discovered in Escherichia coli as an operating system for the bacterial strain, and was later identified in other species. The Pho system is composed of various components including extracellular enzymes and transporters that are capable of phosphate assimilation in addition to extracting inorganic phosphate from organic sources. This is an essential process since phosphate plays an important role in cellular membranes, genetic expression, and metabolism within the cell. Under low nutrient availability, the Pho regulon helps the cell survive and thrive despite a depletion of phosphate within the environment. When this occurs, phosphate starvation-inducible (psi) genes activate other proteins that aid in the transport of inorganic phosphate.
References
- ↑ Shattuck-Eidens, Donna M.; Kadner, Robert J. (1981). "Exogenous Induction of the Escherichia coli Hexose Phosphate Transport System Defined by uhp-lac Operon Fusions". Journal of Bacteriology. 148 (1): 203–9. PMC 216182 . PMID 6793554.
- 1 2 3 Shattuck-Eidens, Donna M.; Kadner, Robert J. (1983). "Molecular Cloning of the uhp Region and Evidence for a Positive Activator for Expression of the Hexose Phosphate Transport System of Escherichia coli". Journal of Bacteriology. 155 (3): 1062–70. PMC 217799 . PMID 6350260.
- 1 2 Hoffer, Sally; Uden, Nathalie; Tommassen, Jan (2001). "Expression of the pho regulon interferes with induction of the uhpT gene in Escherichia coli K-12". Archives of Microbiology. 176 (5): 370–6. doi:10.1007/s002030100339. PMID 11702079.