Sampling

The main sources of tissue sampling are prostatectomy and prostate biopsy.[ citation needed ]
A histopathologic diagnosis of prostate cancer is the discernment of whether there is a cancer in the prostate, as well as specifying any subdiagnosis of prostate cancer if possible. The histopathologic subdiagnosis of prostate cancer has implications for the possibility and methodology of any subsequent Gleason scoring. [1] The most common histopathological subdiagnosis of prostate cancer is acinar adenocarcinoma, constituting 93% of prostate cancers. [2] The most common form of acinar adenocarcinoma, in turn, is "adenocarcinoma, not otherwise specified", also termed conventional, or usual acinar adenocarcinoma. [3]

The main sources of tissue sampling are prostatectomy and prostate biopsy.[ citation needed ]

| Subdiagnosis | Relative incidence | Image | Microscopic characteristics | Immunohistochemistry | Gleason scoring | ||
|---|---|---|---|---|---|---|---|
| Core biopsy | Radical prostatectomy | ||||||
| Acinar adenocarcinoma - 93% [2] | Adenocarcinoma (not otherwise specified/ conventional/ usual acinar) [3] | 77% [notes 2] | 54% [notes 2] | 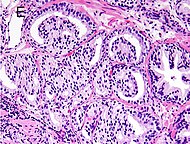 | Further information in section below
| Tumorous glands: | As usual |
| Foamy gland carcinoma | 17% [5] [notes 1] | 13–23% [5] [notes 1] | Based on architecture, discounting foamy cytoplasms [1] | ||||
| Atrophic carcinoma | 2% [5] [notes 3] | 16% [5] [notes 3] | Tumorous glands: | As usual [1] | |||
| Pseudohyperplastic carcinoma | 2% [5] | 11% [5] |
| Tumorous glands: | 3+3=6 [1] | ||
| Microcystic carcinoma | 11% [5] | On (usually) adjacent acinar adeocarcinoma [6] | |||||
| PIN-like | 1.3% [7] |
| Tumorous glands:
| Not recommended [1] | |||
| Non acinar (or mixed acinar/ non-acinar) adenocarcinoma | Ductal adenocarcinoma | 3% to 12.7% [8] [notes 1] |  | ||||
| Intraductal adenocarcinoma | 2.8% [10] | 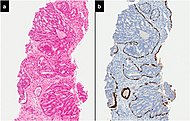 H&E and CK5/6 | |||||
| Urothelial carcinoma | 0.7 to 2.8% [12] |  |
| Not recommended [1] | |||
| Small-cell carcinoma | 0.3–2% [14] [15] [notes 1] | 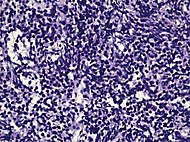 |
Half of cases have usual acinar components [1] | ||||
| Mucinous adenocarcinoma | 0.2% [12] | 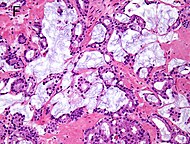 |
| Tumorous glands: | 4+4=8 for irregular cribriform glands floating in mucin. [1] | ||
| Signet-ring adenocarcinoma | 0.02% [16] |  |
| Tumorous glands: | Not recommended [1] | ||
| Basal-cell carcinoma | 0.01% [17] | Basaloid tumor:
BCC-pattern:
| Not recommended. [1] | ||||

In uncertain cases, a diagnosis of malignancy can be excluded by immunohistochemical detection of basal cells (or confirmed by absence thereof), [4] such as using the PIN-4 cocktail of stains, which targets p63, CK-5, CK-14 and AMACR (latter also known as P504S).
Other prostate cancer tumor markers may be necessary in cases that remain uncertain after microscopy.
These constitute 93% of prostate cancers. [2]
In uncertain cases, a diagnosis of malignancy can be discarded by immunohistochemical detection of basal cells. [4]

Intraductal carcinoma of the prostate gland (IDCP), which is now categorised as a distinct entity by WHO 2016, includes two biologically distinct diseases. IDCP associated with invasive carcinoma (IDCP-inv) generally represents a growth pattern of invasive prostatic adenocarcinoma while the rarely encountered pure IDCP is a precursor of prostate cancer. [20] The diagnostic criterion of nuclear size at least 6 times normal is ambiguous as size could refer to either nuclear area or diameter. If area, then this criterion could be re-defined as nuclear diameter at least three times normal as it is difficult to visually compare area of nuclei. [20] It is also unclear whether IDCP could also include tumors with ductal morphology. [20] There is no consensus whether pure IDCP in needle biopsies should be managed with re-biopsy or radical therapy. A pragmatic approach would be to recommend radical therapy only for extensive pure IDCP that is morphologically unequivocal for high-grade prostate cancer. [20] Active surveillance is not appropriate when low-grade invasive cancer is associated with IDCP, as such patients usually have unsampled high-grade prostatic adenocarcinoma. [20] It is generally recommended that IDCP component of IDCP-inv should be included in tumor extent but not grade. [20] However, there are good arguments in favor of grading IDCP associated with invasive cancer. [20] WHO 2016 recommends that IDCP should not be graded, but it is unclear whether this applies to both pure IDCP and IDCP-inv. [20]
Ductal adenocarcinoma may have a prominent cribriforming architecture, with glands appearing relatively round, and may thereby mimic intraductal adenocarcinoma, but can be distinguished by the following features: [10]
| Feature | Ductal adenocarcinoma | Intraductal adenocarcinoma |
|---|---|---|
| True fibrovascular cores in micropapillary architecture | Present | Usually absent |
| Cribriform lumens | Lined by pseudostratified, columnar cells | Punched out lumens lined by cuboidal cells |
| Basal cell markers | Usually negative | Usually positive |
Further workup of a diagnosis of prostate cancer includes mainly:[ citation needed ]
{{cite web}}: CS1 maint: multiple names: authors list (link) Last staff update: 20 November 2023 ![]() This article incorporates text from a free content work. Licensed under Creative Commons Attribution 4.0 International (CC BY 4.0) license.( license statement/permission ). Text taken from adenocarcinoma Prostate adenocarcinoma , Patholines.
This article incorporates text from a free content work. Licensed under Creative Commons Attribution 4.0 International (CC BY 4.0) license.( license statement/permission ). Text taken from adenocarcinoma Prostate adenocarcinoma , Patholines.

Adenocarcinoma is a type of cancerous tumor that can occur in several parts of the body. It is defined as neoplasia of epithelial tissue that has glandular origin, glandular characteristics, or both. Adenocarcinomas are part of the larger grouping of carcinomas, but are also sometimes called by more precise terms omitting the word, where these exist. Thus invasive ductal carcinoma, the most common form of breast cancer, is adenocarcinoma but does not use the term in its name—however, esophageal adenocarcinoma does to distinguish it from the other common type of esophageal cancer, esophageal squamous cell carcinoma. Several of the most common forms of cancer are adenocarcinomas, and the various sorts of adenocarcinoma vary greatly in all their aspects, so that few useful generalizations can be made about them.

Carcinoma is a malignancy that develops from epithelial cells. Specifically, a carcinoma is a cancer that begins in a tissue that lines the inner or outer surfaces of the body, and that arises from cells originating in the endodermal, mesodermal or ectodermal germ layer during embryogenesis.

Invasive carcinoma of no special type, invasive breast carcinoma of no special type (IBC-NST), invasive ductal carcinoma (IDC), infiltrating ductal carcinoma (IDC) or invasive ductal carcinoma, not otherwise specified (NOS) is a disease. For international audiences this article will use "invasive carcinoma NST" because it is the preferred term of the World Health Organization (WHO).

The Gleason grading system is used to help evaluate the prognosis of men with prostate cancer using samples from a prostate biopsy. Together with other parameters, it is incorporated into a strategy of prostate cancer staging which predicts prognosis and helps guide therapy. A Gleason score is given to prostate cancer based upon its microscopic appearance.

Ductal carcinoma in situ (DCIS), also known as intraductal carcinoma, is a pre-cancerous or non-invasive cancerous lesion of the breast. DCIS is classified as Stage 0. It rarely produces symptoms or a breast lump that can be felt, typically being detected through screening mammography. It has been diagnosed in a significant percentage of men.

Tumor protein p63, typically referred to as p63, also known as transformation-related protein 63 is a protein that in humans is encoded by the TP63 gene.

Salivary gland tumours, also known as mucous gland adenomas or neoplasms, are tumours that form in the tissues of salivary glands. The salivary glands are classified as major or minor. The major salivary glands consist of the parotid, submandibular, and sublingual glands. The minor salivary glands consist of 800 to 1000 small mucus-secreting glands located throughout the lining of the oral cavity. Patients with these types of tumours may be asymptomatic.
Comedocarcinoma is a kind of breast cancer that demonstrates comedonecrosis, which is the central necrosis of cancer cells within involved ducts. Comedocarcinomas are usually non-infiltrating and intraductal tumors, characterized as a comedo-type, high-grade ductal carcinoma in situ (DCIS). However, there have been accounts of comedocarcinoma which has then diversified into other cell types and developed into infiltrating (invasive) ductal carcinoma. Recurrence and survival rates differ for invasive breast cancer which has originated as comedocarcinoma compared with other types of cancer cells.

Lobular carcinoma in situ (LCIS) is an incidental microscopic finding with characteristic cellular morphology and multifocal tissue patterns. The condition is a laboratory diagnosis and refers to unusual cells in the lobules of the breast. The lobules and acini of the terminal duct-lobular unit (TDLU), the basic functional unit of the breast, may become distorted and undergo expansion due to the abnormal proliferation of cells comprising the structure. These changes represent a spectrum of atypical epithelial lesions that are broadly referred to as lobular neoplasia (LN).
In urologic pathology, atypical small acinar proliferation, is a collection of small prostatic glands, on prostate biopsy, whose significance is uncertain and cannot be determined to be benign or malignant.

High-grade prostatic intraepithelial neoplasia (HGPIN) is an abnormality of prostatic glands and believed to precede the development of prostate adenocarcinoma.

Atypical ductal hyperplasia (ADH) is the term used for a benign lesion of the breast that indicates an increased risk of breast cancer.
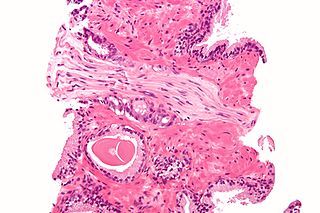
In pathology, perineural invasion, abbreviated PNI, refers to the invasion of cancer to the space surrounding a nerve. It is common in head and neck cancer, prostate cancer and colorectal cancer.

Adenocarcinoma of the lung is the most common type of lung cancer, and like other forms of lung cancer, it is characterized by distinct cellular and molecular features. It is classified as one of several non-small cell lung cancers (NSCLC), to distinguish it from small cell lung cancer which has a different behavior and prognosis. Lung adenocarcinoma is further classified into several subtypes and variants. The signs and symptoms of this specific type of lung cancer are similar to other forms of lung cancer, and patients most commonly complain of persistent cough and shortness of breath.
Acinar adenocarcinoma is a histological subtype of gland-forming cancer that is diagnosed when cuboidal and/or columnar shaped malignant cells in the neoplastic tissue form acini and tubules. It is a common form of cancer occurring in the lung and prostate gland.
Neuroendocrine differentiation is a term primarily used in relation to prostate cancers that display a significant neuroendocrine cell population on histopathological examination. These types of prostate cancer comprise true neuroendocrine cancers, such as small cell carcinoma, carcinoid and carcinoid-like tumors, as well as prostatic adenocarcinoma exhibiting focal neuroendocrine phenotype.
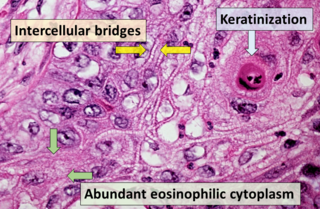
Squamous-cell carcinoma (SCC), also known as epidermoid carcinoma, comprises a number of different types of cancer that begin in squamous cells. These cells form on the surface of the skin, on the lining of hollow organs in the body, and on the lining of the respiratory and digestive tracts.

The histopathology of colorectal cancer of the adenocarcinoma type involves analysis of tissue taken from a biopsy or surgery. A pathology report contains a description of the microscopical characteristics of the tumor tissue, including both tumor cells and how the tumor invades into healthy tissues and finally if the tumor appears to be completely removed. The most common form of colon cancer is adenocarcinoma, constituting between 95% and 98% of all cases of colorectal cancer. Other, rarer types include lymphoma, adenosquamous and squamous cell carcinoma. Some subtypes have been found to be more aggressive.
Papillary carcinomas of the breast (PCB), also termed malignant papillary carcinomas of the breast, are rare forms of the breast cancers. The World Health Organization (2019) classified papillary neoplasms of the breast into 5 types: intraductal papilloma, papillary ductal carcinoma in situ (PDCIS), encapsulated papillary carcinoma (EPC), solid-papillary carcinoma (SPC), and invasive papillary carcinoma (IPC). The latter four carcinomas are considered here; intraductal papilloma is a benign neoplasm. The World Health Organization regarded solid papillary carcinoma as having two subtypes: in situ and invasive SPC.

Invasive cribriform carcinoma of the breast (ICCB), also termed invasive cribriform carcinoma, is a rare type of breast cancer that accounts for 0.3% to 0.6% of all carcinomas in the breast. It originates in a lactiferous duct as opposed to the lobules that form the alveoli in the breasts' mammary glands. ICCB was first described by Dixon and colleagues in 1983 as a tumor that on microscopic histopathological inspection had a cribriform pattern, i.e. a tissue pattern consisting of numerous "Swiss cheese"-like open spaces and/or sieve-like small holes. The latest edition (2019) of the World Health Organization (2019) termed these lesions invasive cribriform carcinomas indicating that by definition they must have a component that invades out of their ducts of origin into adjacent tissues. In situ ductal cancers that have a cribriform histopathology are regarded as belonging to the group of ductal carcinoma in situ tumors.