
An antibiotic is a type of antimicrobial substance active against bacteria and is the most important type of antibacterial agent for fighting bacterial infections. Antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza ; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics.
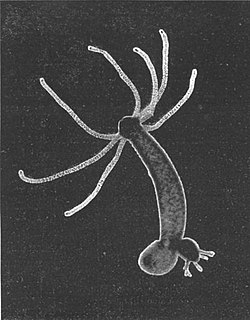
Hydra is a genus of small, fresh-water organisms of the phylum Cnidaria and class Hydrozoa. They are native to the temperate and tropical regions. Biologists are especially interested in Hydra because of their regenerative ability – they do not appear to die of old age, or indeed to age at all.
Peptidoglycan(murein) is a polymer consisting of sugars and amino acids that forms a mesh-like layer outside the plasma membrane of most bacteria, forming the cell wall. The sugar component consists of alternating residues of β-(1,4) linked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). Attached to the N-acetylmuramic acid is a peptide chain of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. Peptidoglycan is also involved in binary fission during bacterial cell reproduction.

Drug resistance is the reduction in effectiveness of a medication such as an antimicrobial or an antineoplastic in treating a disease or condition. The term is used in the context of resistance that pathogens or cancers have "acquired", that is, resistance has evolved. Antimicrobial resistance and antineoplastic resistance challenge clinical care and drive research. When an organism is resistant to more than one drug, it is said to be multidrug-resistant.
Aminoglycoside is a medicinal and bacteriologic category of traditional Gram-negative antibacterial medications that inhibit protein synthesis and contain as a portion of the molecule an amino-modified glycoside (sugar). The term can also refer more generally to any organic molecule that contains amino sugar substructures. Aminoglycoside antibiotics display bactericidal activity against Gram-negative aerobes and some anaerobic bacilli where resistance has not yet arisen but generally not against Gram-positive and anaerobic Gram-negative bacteria.

Antimicrobial peptides (AMPs), also called host defense peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.

Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.
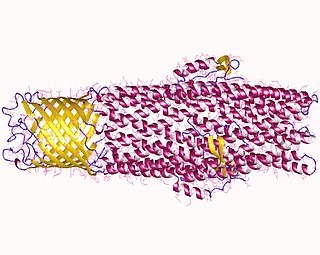
All microorganisms, with a few exceptions, have highly conserved DNA sequences in their genome that are transcribed and translated to efflux pumps. Efflux pumps are capable of moving a variety of different toxic compounds out of cells, such as antibiotics, heavy metals, organic pollutants, plant-produced compounds, quorum sensing signals, bacterial metabolites and neurotransmitters via active efflux, which is vital part for xenobiotic metabolism. This active efflux mechanism is responsible for various types of resistance to bacterial pathogens within bacterial species - the most concerning being antibiotic resistance because microorganisms can have adapted efflux pumps to divert toxins out of the cytoplasm and into extracellular media.
2-Oxazolidone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring.

Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternate mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins.
In enzymology, an L-amino acid oxidase (LAAO) (EC 1.4.3.2) is an enzyme that catalyzes the chemical reaction
Antimicrobial pharmacodynamics is the relationship between concentration of antibiotic and its ability to inhibit vital processes of endo- or ectoparasites and microbial organisms. This branch of pharmacodynamics relates concentration of an anti-infective agent to effect, but specifically to its antimicrobial effect.

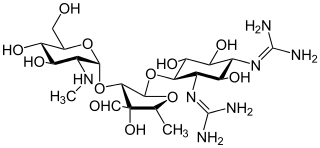
Katanosins are a group of antibiotics. They are natural products with strong antibacterial potency. So far, katanosin A and katanosin B (lysobactin) have been described.

Polylysine refers to several types of lysine homopolymers, which may differ from each other in terms of stereochemistry and link position.

Cecropins are antimicrobial peptides. They were first isolated from the hemolymph of Hyalophora cecropia, whence the term cecropin was derived. Cecropins lyse bacterial cell membranes; they also inhibit proline uptake and cause leaky membranes.

Cnidarians such as Hydra have become attractive model organisms to study the evolution of immunity. However, despite long-term efforts, stably transgenic animals could not be generated, severely limiting the functional analysis of genes. For analytical purposes, therefore, an important technical breakthrough in the field was the development of a transgenic procedure for generation of stably transgenic lines by embryo microinjection.
Theta-defensins are a family of mammalian antimicrobial peptides. They are found in non-human 'Old World' primates, but not in human, gorilla, bonobo, and chimpanzee.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.
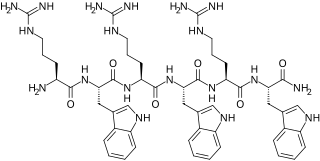
MP196 is a synthetic antimicrobial peptide. It falls under the structural class: short cationic peptides. Since it is a short cationic peptide, it can be easily synthesized, derivatized and isolated. MP196 is rich in tryptophan, a hydrophobic amino acid and arginine residues, a positively charged amino acid. It has structure: RWRWRW-NH2. This a short linear peptide with minimal pharmacophore. MP196 is effective against gram-positive bacteria and moderately effective against gram-negative bacteria.
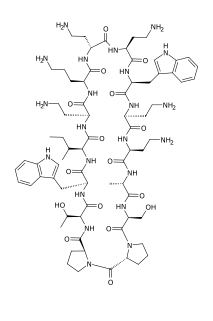
Murepavadin also known as POL7080 is a Pseudomonas specific peptidomimetic antibiotic. It is a synthetic cyclic beta hairpin peptidomimetic based on the cationic antimicrobial peptide protegrin I (PG-1) and the first example of an outer membrane protein-targeting antibiotic class with a novel, nonlytic mechanism of action, highly active and selective against the protein transporter LptD of Pseudomonas aeruginosa. In preclinical studies the compound was highly active on a broad panel of clinical isolates including multi-drug resistant Pseudomonas bacteria with outstanding in vivo efficacy in sepsis, lung, and thigh infection models. Intravenous murepavadin is in development for the treatment of bacterial hospital-acquired pneumonia and bacterial ventilator-associated pneumonia due to Pseudomonas aeruginosa.















