Related Research Articles

Xylem is one of the two types of transport tissue in vascular plants, the other being phloem. The basic function of xylem is to transport water from roots to stems and leaves, but it also transports nutrients. The word xylem is derived from the Ancient Greek word ξύλον (xylon), meaning "wood"; the best-known xylem tissue is wood, though it is found throughout a plant. The term was introduced by Carl Nägeli in 1858.

In vascular plants, the roots are the organs of a plant that are modified to provide anchorage for the plant and take in water and nutrients into the plant body, which allows plants to grow taller and faster. They are most often below the surface of the soil, but roots can also be aerial or aerating, that is, growing up above the ground or especially above water.

A mycorrhiza is a symbiotic association between a fungus and a plant. The term mycorrhiza refers to the role of the fungus in the plant's rhizosphere, its root system. Mycorrhizae play important roles in plant nutrition, soil biology, and soil chemistry.
In botany, an evergreen is a plant which has foliage that remains green and functional through more than one growing season. This contrasts with deciduous plants, which completely lose their foliage during the winter or dry season.

Vascular plants, also called tracheophytes or collectively Tracheophyta, form a large group of land plants that have lignified tissues for conducting water and minerals throughout the plant. They also have a specialized non-lignified tissue to conduct products of photosynthesis. Vascular plants include the clubmosses, horsetails, ferns, gymnosperms, and angiosperms. Scientific names for the group include Tracheophyta, Tracheobionta and Equisetopsida sensu lato. Some early land plants had less developed vascular tissue; the term eutracheophyte has been used for all other vascular plants, including all living ones.
Soil formation, also known as pedogenesis, is the process of soil genesis as regulated by the effects of place, environment, and history. Biogeochemical processes act to both create and destroy order (anisotropy) within soils. These alterations lead to the development of layers, termed soil horizons, distinguished by differences in color, structure, texture, and chemistry. These features occur in patterns of soil type distribution, forming in response to differences in soil forming factors.

Root pressure is the transverse osmotic pressure within the cells of a root system that causes sap to rise through a plant stem to the leaves.
Soil moisture is the water content of the soil. It can be expressed in terms of volume or weight. Soil moisture measurement can be based on in situ probes or remote sensing methods.
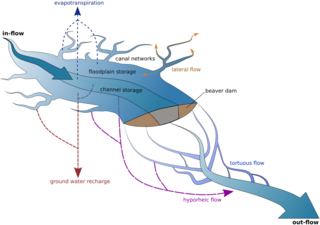
Ecohydrology is an interdisciplinary scientific field studying the interactions between water and ecological systems. It is considered a sub discipline of hydrology, with an ecological focus. These interactions may take place within water bodies, such as rivers and lakes, or on land, in forests, deserts, and other terrestrial ecosystems. Areas of research in ecohydrology include transpiration and plant water use, adaption of organisms to their water environment, influence of vegetation and benthic plants on stream flow and function, and feedbacks between ecological processes, the soil carbon sponge and the hydrological cycle.
Moisture stress is a form of abiotic stress that occurs when the moisture of plant tissues is reduced to suboptimal levels. Water stress occurs in response to atmospheric and soil water availability when the transpiration rate exceeds the rate of water uptake by the roots and cells lose turgor pressure. Moisture stress is described by two main metrics, water potential and water content.

Saururus cernuus is a medicinal and ornamental plant native to eastern North America. It grows in wet areas or shallow water, and can be up to about a meter tall. The native range covers much of the eastern United States, as far west as eastern Texas and Kansas, south to Florida, and north to Michigan and New York state. Saururus cernuus also occurs in Ontario Canada. It is an obligate wetland plant and able to grow in saturated soils.

In ecology, a mesic habitat is a type of habitat with a well-balanced supply of moisture throughout the growing season, e.g., a mesic forest, a temperate hardwood forest, or dry-mesic prairie. Mesic is one of a triad of terms used to label a habitat or area that has a moderate or well-balanced supply of water within.

Prosopis tamarugo, commonly known as the tamarugo, is a species of flowering tree in the pea family, Fabaceae, subfamilia Mimosoideae. It is only found in northern Chile, particularly in the Pampa del Tamarugal, some 70 km (43 mi) east of the city of Iquique. This bushy tree apparently grows without the benefit of rainfall, and it is thought to obtain some water from dew. Studies indicate it is a Phreatophyte; having deep roots that tap into ground water supplies. It also participates in hydraulic redistribution moving water from deeper levels to the upper and also reversing the process in times of severe drought.

Sedum album, the white stonecrop, is a flowering plant of the genus Sedum in the family Crassulaceae. It is found in the northern temperate regions of the world, often growing in crevices or free-draining rocky soil. As a long-day plant it grows vegetatively for most of the year and flowers in summer.
A xerophyte is a species of plant that has adaptations to survive in an environment with little liquid water. Examples are typically desert regions like the Sahara, and places in the Alps or the Arctic. Popular examples of xerophytes are cacti, pineapple and some Gymnosperm plants.

Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. Water is necessary for plants but only a small amount of water taken up by the roots is used for growth and metabolism. The remaining 97–99.5% is lost by transpiration and guttation. Leaf surfaces are dotted with pores called stomata, and in most plants they are more numerous on the undersides of the foliage. The stomata are bordered by guard cells and their stomatal accessory cells that open and close the pore. Transpiration occurs through the stomatal apertures, and can be thought of as a necessary "cost" associated with the opening of the stomata to allow the diffusion of carbon dioxide gas from the air for photosynthesis. Transpiration also cools plants, changes osmotic pressure of cells, and enables mass flow of mineral nutrients and water from roots to shoots. Two major factors influence the rate of water flow from the soil to the roots: the hydraulic conductivity of the soil and the magnitude of the pressure gradient through the soil. Both of these factors influence the rate of bulk flow of water moving from the roots to the stomatal pores in the leaves via the xylem.
Specific leaf area (SLA) is the ratio of leaf area to leaf dry mass. The inverse of SLA is Leaf Mass per Area (LMA).
Biomass partitioning is the process by which plants divide their energy among their leaves, stems, roots, and reproductive parts. These four main components of the plant have important morphological roles: leaves take in CO2 and energy from the sun to create carbon compounds, stems grow above competitors to reach sunlight, roots absorb water and mineral nutrients from the soil while anchoring the plant, and reproductive parts facilitate the continuation of species. Plants partition biomass in response to limits or excesses in resources like sunlight, carbon dioxide, mineral nutrients, and water and growth is regulated by a constant balance between the partitioning of biomass between plant parts. An equilibrium between root and shoot growth occurs because roots need carbon compounds from photosynthesis in the shoot and shoots need nitrogen absorbed from the soil by roots. Allocation of biomass is put towards the limit to growth; a limit below ground will focus biomass to the roots and a limit above ground will favor more growth in the shoot.

Nurse plants are plants that serve the ecological role of helping seedlings establish themselves and protecting young plants from harsh conditions. This effect is particularly well studied among plant communities in xeric environments.
Nina Buchmann is a German ecologist known for her research on the physiology of plants and the impact of plants on biogeochemical cycling. She is a member of the German National Academy of Sciences Leopoldina and an elected fellow of the American Geophysical Union.
References
- 1 2 Allen, Michael F.; Vargas, Rodrigo; Prieto, Iván; Egerton-Warburton, Louise M.; Querejeta, José Ignacio (2012-06-01). "Changes in soil hyphal abundance and viability can alter the patterns of hydraulic redistribution by plant roots". Plant and Soil. 355 (1–2): 63–73. doi:10.1007/s11104-011-1080-8. ISSN 1573-5036. S2CID 15742304.
- 1 2 3 4 Meinzer, Frederick C.; Clearwater, Michael J.; Goldstein, Guillermo (2001-06-01). "Water transport in trees: current perspectives, new insights and some controversies". Environmental and Experimental Botany. 45 (3): 239–262. doi:10.1016/S0098-8472(01)00074-0. ISSN 0098-8472. PMID 11323032.
- 1 2 3 4 5 Querejeta, José I.; Navarro-Cano, José A.; Alguacil, María del Mar; Huygens, Dries; Roldán, Antonio; Prieto, Iván (2016-09-01). "Species-specific roles of ectomycorrhizal fungi in facilitating interplant transfer of hydraulically redistributed water between Pinus halepensis saplings and seedlings". Plant and Soil. 406 (1–2): 15–27. doi:10.1007/s11104-016-2860-y. ISSN 1573-5036. S2CID 18442276.
- 1 2 Simard, Suzanne; Bingham, Marcus A. (2012-03-01). "Ectomycorrhizal Networks of Pseudotsuga menziesii var. glauca Trees Facilitate Establishment of Conspecific Seedlings Under Drought". Ecosystems. 15 (2): 188–199. doi:10.1007/s10021-011-9502-2. ISSN 1435-0629. S2CID 17432918.
- ↑ Burgess, S. S. O.; Adams, M. A.; Turner, N. C.; Beverly, C. R.; Ong, C. K.; Khan, A. A. H.; Bleby, T. M. (2001). "An improved heat pulse method to measure low and reverse rates of sap flow in woody plants". Tree Physiology. 21 (9): 589–598. doi: 10.1093/treephys/21.9.589 . PMID 11390303.
- 1 2 Richards, J. H.; Caldwell, M. M. (1987). "Hydraulic lift: Substantial nocturnal water transport between soil layers by Artemisia tridentata roots". Oecologia. 73 (4): 486–489. Bibcode:1987Oecol..73..486R. doi:10.1007/BF00379405. PMID 28311963. S2CID 40289775.
- 1 2 Smart, D. R.; Carlisle, E.; Goebel, M.; Nunez, B. A. (2005). "Transverse hydraulic redistribution by a grapevine". Plant, Cell and Environment. 28 (2): 157–166. doi: 10.1111/j.1365-3040.2004.01254.x .
- 1 2 3 Oliveira, Rafael S.; Dawson, Todd E.; Burgess, Stephen S. O.; Nepstad, Daniel C. (2005). "Hydraulic redistribution in three Amazonian trees". Oecologia. 145 (3): 354–363. Bibcode:2005Oecol.145..354O. doi:10.1007/s00442-005-0108-2. PMID 16091971. S2CID 25867083.
- 1 2 3 4 Dawson, Todd E. (1993). "Hydraulic lift and water use by plants: Implications for water balance, performance and plant-plant interactions". Oecologia. 95 (4): 565–574. Bibcode:1993Oecol..95..565D. doi:10.1007/BF00317442. PMID 28313298. S2CID 30249552.
- ↑ Schulze, E.-D.; Caldwell, M. M.; Canadell, J.; Mooney, H. A.; Jackson, R. B.; Parson, D.; Scholes, R.; Sala, O. E.; Trimborn, P. (1998). "Downward flux of water through roots (i.e. Inverse hydraulic lift) in dry Kalahari sands". Oecologia. 115 (4): 460–462. Bibcode:1998Oecol.115..460S. doi:10.1007/s004420050541. PMID 28308264. S2CID 22181427.
- 1 2 Burgess, S.; Bleby, T. M. (2006). "Redistribution of soil water by lateral roots mediated by stem tissues". Journal of Experimental Botany. 57 (12): 3283–3291. doi: 10.1093/jxb/erl085 . PMID 16926237.
- 1 2 Schulze, E. -D; Caldwell, M. M.; Canadell, J.; Mooney, H. A.; Jackson, R. B.; Parson, D.; Scholes, R.; Sala, O. E.; Trimborn, P. (1998). "Downward flux of water through roots (i.e. Inverse hydraulic lift) in dry Kalahari sands". Oecologia. 115 (4): 460–462. Bibcode:1998Oecol.115..460S. doi:10.1007/s004420050541. PMID 28308264. S2CID 22181427.
- ↑ Leffler, A. Joshua; Peek, Michael S.; Ryel, Ron J.; Ivans, Carolyn Y.; Caldwell, Martyn M. (2005). "Hydraulic Redistribution Through the Root Systems of Senesced Plants". Ecology. 86 (3): 633–642. doi:10.1890/04-0854.
- 1 2 Allen, Michael F. (2009). "Bidirectional water flows through the soil–fungal–plant mycorrhizal continuum". New Phytologist. 182 (2): 290–293. doi: 10.1111/j.1469-8137.2009.02815.x . ISSN 1469-8137. PMID 19338631.
- ↑ Caldwell, Martyn M.; Dawson, Todd E.; Richards, James H. (1998). "Hydraulic lift: Consequences of water efflux from the roots of plants". Oecologia. 113 (2): 151–161. Bibcode:1998Oecol.113..151C. doi:10.1007/s004420050363. PMID 28308192. S2CID 24181646.
- ↑ Ryel, R.; Caldwell, M.; Yoder, C.; Or, D.; Leffler, A. (2002). "Hydraulic redistribution in a stand of Artemisia tridentata: Evaluation of benefits to transpiration assessed with a simulation model". Oecologia. 130 (2): 173–184. Bibcode:2002Oecol.130..173R. doi:10.1007/s004420100794. PMID 28547139. S2CID 21154225.
- ↑ Brooks, J. R.; Meinzer, F. C.; Coulombe, R.; Gregg, J. (2002). "Hydraulic redistribution of soil water during summer drought in two contrasting Pacific Northwest coniferous forests". Tree Physiology. 22 (15–16): 1107–1117. doi: 10.1093/treephys/22.15-16.1107 . PMID 12414370.
- ↑ Burgess, Stephen S. O.; Adams, Mark A.; Turner, Neil C.; Ong, Chin K. (1998). "The redistribution of soil water by tree root systems". Oecologia. 115 (3): 306–311. Bibcode:1998Oecol.115..306B. doi:10.1007/s004420050521. PMID 28308420. S2CID 19978719.
- ↑ Meinzer, F. C.; James, S. A.; Goldstein, G. (2004). "Dynamics of transpiration, sap flow and use of stored water in tropical forest canopy trees". Tree Physiology. 24 (8): 901–909. doi: 10.1093/treephys/24.8.901 . PMID 15172840.
- ↑ Neumann, Rebecca B.; Cardon, Zoe G. (2012). "The magnitude of hydraulic redistribution by plant roots: A review and synthesis of empirical and modeling studies". New Phytologist. 194 (2): 337–352. doi: 10.1111/j.1469-8137.2012.04088.x . PMID 22417121.