Related Research Articles

Hydrogen peroxide is a chemical compound with the formula H2O2. In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution in water for consumer use and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used as both a monopropellant and an oxidizer in rocketry.

In chemistry, pH, also referred to as acidity or basicity, historically denotes "potential of hydrogen". It is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Acidic solutions are measured to have lower pH values than basic or alkaline solutions.
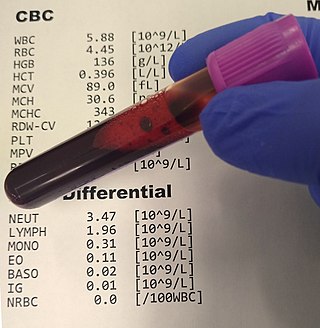
A complete blood count (CBC), also known as a full blood count (FBC), is a set of medical laboratory tests that provide information about the cells in a person's blood. The CBC indicates the counts of white blood cells, red blood cells and platelets, the concentration of hemoglobin, and the hematocrit. The red blood cell indices, which indicate the average size and hemoglobin content of red blood cells, are also reported, and a white blood cell differential, which counts the different types of white blood cells, may be included.
A hydrogen ion is created when a hydrogen atom loses an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space. Due to its extremely high charge density of approximately 2×1010 times that of a sodium ion, the bare hydrogen ion cannot exist freely in solution as it readily hydrates, i.e., bonds quickly. The hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes. Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions.

The mean corpuscular hemoglobin concentration (MCHC) is a measure of the concentration of hemoglobin in a given volume of packed red blood cell.
High-test peroxide (HTP) is a highly concentrated solution of hydrogen peroxide, with the remainder consisting predominantly of water. In contact with a catalyst, it decomposes into a high-temperature mixture of steam and oxygen, with no remaining liquid water. It was used as a propellant of HTP rockets and torpedoes, and has been used for high-performance vernier engines.

Elemental analysis is a process where a sample of some material is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualitative, and it can be quantitative. Elemental analysis falls within the ambit of analytical chemistry, the instruments involved in deciphering the chemical nature of our world.

A breathalyzer or breathalyser is a device for measuring breath alcohol content (BrAC). The name is a genericized trademark of the Breathalyzer brand name of instruments developed by inventor Robert Frank Borkenstein in the 1950s.
The AutoAnalyzer is an automated analyzer using a flow technique called continuous flow analysis (CFA), or more correctly segmented flow analysis (SFA) first made by the Technicon Corporation. The instrument was invented in 1957 by Leonard Skeggs, PhD and commercialized by Jack Whitehead's Technicon Corporation. The first applications were for clinical analysis, but methods for industrial and environmental analysis soon followed. The design is based on segmenting a continuously flowing stream with air bubbles.

A polarimeter is a scientific instrument used to measure optical rotation: the angle of rotation caused by passing linearly polarized light through an optically active substance.
Combustion analysis is a method used in both organic chemistry and analytical chemistry to determine the elemental composition of a pure organic compound by combusting the sample under conditions where the resulting combustion products can be quantitatively analyzed. Once the number of moles of each combustion product has been determined the empirical formula or a partial empirical formula of the original compound can be calculated.
Hydrogen leak testing is the normal way in which a hydrogen pressure vessel or installation is checked for leaks or flaws. This usually involves charging hydrogen as a tracer gas into the device undergoing testing, with any leaking gas detected by hydrogen sensors. Various test mechanisms have been devised.
Moisture analysis covers a variety of methods for measuring the moisture content in solids, liquids, or gases. For example, moisture is a common specification in commercial food production. There are many applications where trace moisture measurements are necessary for manufacturing and process quality assurance. Trace moisture in solids must be known in processes involving plastics, pharmaceuticals and heat treatment. Fields that require moisture measurement in gasses or liquids include hydrocarbon processing, pure semiconductor gases, bulk pure or mixed gases, dielectric gases such as those in transformers and power plants, and natural gas pipeline transport. Moisture content measurements can be reported in multiple units, such as: parts per million, pounds of water per million standard cubic feet of gas, mass of water vapor per unit volume or mass of water vapor per unit mass of dry gas.
Continuous emission monitoring systems (CEMS) are used as a tool to monitor the effluent gas streams resulting from combustion in industrial processes. CEMS can measure flue gas for oxygen, carbon monoxide and carbon dioxide to provide information for combustion control in industrial settings. They are also used as a means to comply with air emission standards such as the United States Environmental Protection Agency's (EPA) Acid Rain Program, other US federal emission programs, or state permitted emission standards. CEMS typically consist of analyzers to measure gas concentrations within the stream, equipment to direct a sample of that gas stream to the analyzers if they are remote, equipment to condition the sample gas by removing water and other components that could interfere with the reading, pneumatic plumbing with valves that can be controlled by a PLC to route the sample gas to and away from the analyzers, a calibration and maintenance system that allows for the injection of calibration gases into the sample line, and a Data Acquisition and Handling System (DAHS) that collects and stores each data point and can perform necessary calculations required to get total mass emissions. A CEMS operates at all times even if the process it measures is not on. They can continuously collect, record and report emissions data for process monitoring and/or for compliance purposes.
Vaporized hydrogen peroxide (trademarked VHP, also known as hydrogen peroxide vapor, HPV) is a vapor form of hydrogen peroxide (H2O2) with applications as a low-temperature antimicrobial vapor used to decontaminate enclosed and sealed areas such as laboratory workstations, isolation and pass-through rooms, and even aircraft interiors.

Explorer 54, also called as AE-D, was a NASA scientific satellite belonging to series Atmosphere Explorer, being launched on 6 October 1975 from Vandenberg Air Force Base board a Thor-Delta 2910 launch vehicle.
Hydrogen gas porosity is an aluminium casting defect in the form of a porosity or void in an aluminium casting caused by a high level of hydrogen gas (H2) dissolved in the aluminium at liquid phase. The solubility of hydrogen in solid aluminium is much smaller than in liquid aluminium. As the aluminium freezes, some of the hydrogen comes out of solution and forms bubbles, creating porosity in the solid aluminium.
Colorimetric analysis is a method of determining the concentration of a chemical element or chemical compound in a solution with the aid of a color reagent. It is applicable to both organic compounds and inorganic compounds and may be used with or without an enzymatic stage. The method is widely used in medical laboratories and for industrial purposes, e.g. the analysis of water samples in connection with industrial water treatment.

A Helium analyzer is an instrument used to identify the presence and concentration of helium in a mixture of gases. In Technical diving where breathing gas mixtures known as Trimix comprising oxygen, helium and nitrogen are used, it is necessary to know the fraction of helium in the mixture to reliably calculate decompression schedules for dives using that mixture.
Explorer 55, also called as AE-E, was a NASA scientific satellite belonging to series Atmosphere Explorer, being launched on 20 November 1975 from Cape Canaveral Air Force Station (CCAFS) board a Thor-Delta 2910 launch vehicle.
References
- ↑ "Hydrogen Analyzer". www.labcompare.com. Retrieved April 7, 2024.