
Ulcerative colitis (UC) is a long-term condition that results in inflammation and ulcers of the colon and rectum. The primary symptoms of active disease are abdominal pain and diarrhea mixed with blood (hematochezia). Weight loss, fever, and anemia may also occur. Often, symptoms come on slowly and can range from mild to severe. Symptoms typically occur intermittently with periods of no symptoms between flares. Complications may include abnormal dilation of the colon (megacolon), inflammation of the eye, joints, or liver, and colon cancer.

Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the colon and small intestine, Crohn's disease and ulcerative colitis (UC) being the principal types. Crohn's disease affects the small intestine and large intestine, as well as the mouth, esophagus, stomach and the anus, whereas ulcerative colitis primarily affects the colon and the rectum.

Allergic conjunctivitis (AC) is inflammation of the conjunctiva due to allergy. Although allergens differ among patients, the most common cause is hay fever. Symptoms consist of redness, edema (swelling) of the conjunctiva, itching, and increased lacrimation. If this is combined with rhinitis, the condition is termed allergic rhinoconjunctivitis (ARC).
Leukapheresis is a laboratory procedure in which white blood cells are separated from a sample of blood. It is a specific type of apheresis, the more general term for separating out one particular constituent of blood and returning the remainder to the circulation.
Pouchitis is an umbrella term for inflammation of the ileal pouch, an artificial rectum surgically created out of ileum in patients who have undergone a proctocolectomy or total colectomy. The ileal pouch-anal anastomosis is created in the management of patients with ulcerative colitis, indeterminate colitis, familial adenomatous polyposis, cancer, or rarely, other colitides.

Biological therapy, the use of medications called biopharmaceuticals or biologics that are tailored to specifically target an immune or genetic mediator of disease, plays a major role in the treatment of inflammatory bowel disease. Even for diseases of unknown cause, molecules that are involved in the disease process have been identified, and can be targeted for biological therapy. Many of these molecules, which are mainly cytokines, are directly involved in the immune system. Biological therapy has found a niche in the management of cancer, autoimmune diseases, and diseases of unknown cause that result in symptoms due to immune related mechanisms.

Chemokine ligand 2 (CXCL2) is a small cytokine belonging to the CXC chemokine family that is also called macrophage inflammatory protein 2-alpha (MIP2-alpha), Growth-regulated protein beta (Gro-beta) and Gro oncogene-2 (Gro-2). CXCL2 is 90% identical in amino acid sequence as a related chemokine, CXCL1. This chemokine is secreted by monocytes and macrophages and is chemotactic for polymorphonuclear leukocytes and hematopoietic stem cells. The gene for CXCL2 is located on human chromosome 4 in a cluster of other CXC chemokines. CXCL2 mobilizes cells by interacting with a cell surface chemokine receptor called CXCR2.

Biotie Therapies was a Finnish biotechnology and pharmaceutics company that was acquired by Acorda Therapeutics in January 2016. The company's research and development was focused on drugs for neurodegenerative and psychiatric disorders like Parkinson's disease, Alzheimer's disease and other cognitive disorders, alcohol and drug dependence and post traumatic stress disorder, and inflammatory and fibrotic liver disease. The company's headquarters is in Turku, Western Finland, and it is listed on NASDAQ OMX Helsinki.

Losmapimod (GW856553X) is an investigational drug being developed by Fulcrum Therapeutics for the treatment of facioscapulohumeral muscular dystrophy (FSHD); a phase III clinical trial is pending approval. Losmapimod selectively inhibits enzymes p38α/β mitogen-activated protein kinases (MAPKs), which are modulators of DUX4 expression and mediators of inflammation.

Cellectis is a French biopharmaceutical company. It develops genome-edited chimeric antigen receptor T-cell technologies for cancer immunotherapy. It has offices in Paris, New York City, and Raleigh, North Carolina.

Cenicriviroc is an experimental drug candidate for the treatment of HIV infection and in combination with Tropifexor for non-alcoholic steatohepatitis. It is being developed by Takeda and Tobira Therapeutics.
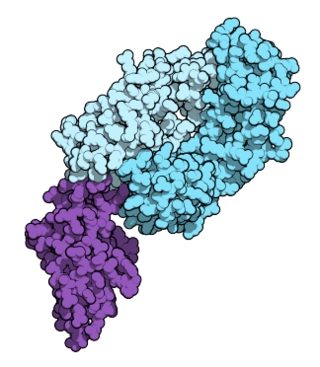
Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.

Alicaforsen is an antisense oligonucleotide therapeutic that targets the messenger RNA for the production of human ICAM-1 receptor and is being developed for the treatment of acute disease flares in moderate to severe Inflammatory Bowel Disease (IBD).
Spotlight Innovation Inc. is an American pharmaceutical holding company. The company currently maintains two subsidiaries: Caretta Therapeutics, Inc. and Celtic Biotech Iowa, Inc. Spotlight Innovation Inc. is based in Urbandale, Iowa and is publicly traded on the OTCQB marketplace under the stock ticker symbol, STLT.

SOTIO Biotech is a Czech biotechnology company focused on clinical-stage research and development of innovative medicines for cancer with operations in Europe, North America, and Asia. The company has clinical programs which include a superagonist of the immuno-oncology target IL-15, a new generation of potent and stable antibody-drug conjugates (ADCs), proprietary technology designed to improve on the efficacy of CAR T therapies and a platform to streamline and enhance personalized cell therapies.
Enadenotucirev is an investigational oncolytic virus that is in clinical trials for various cancers.
Leukocyte apheresis is a medical device therapy for the treatment of inflammation of the colon. It works by removing from the blood a group of white blood cells called activated leukocytes that play a key role in the inflammatory stages of ulcerative colitis (UC). Selectively reducing these cells in the blood helps to reduce inflammation in the colon. Leukocyte apheresis can help UC patients with chronic, grumbling disease who are either unsuitable for, intolerant of, or failing on medicines described above.
AMG 319 is a drug developed by Amgen which acts as an inhibitor of the phosphoinositide 3-kinase enzyme subtype PI3Kδ. It was originally developed as an anti-inflammatory drug with potential applications in the treatment of autoimmune conditions such as rheumatoid arthritis, but subsequent research showed that it inhibits cell proliferation and might potentially have useful anti-cancer effects, and it has been put into clinical trials to assess its safety and tolerability in this application.
Otilimab is a fully human antibody which has been developed by the biotechnology company MorphoSys. It can also be referred to as HuCAL antibody, HuCAL standing for Human Combinatorial Antibody Library and being a technology used to generate monoclonal antibodies. Otilimab is directed against the granulocyte-macrophage colony stimulating factor (GM-CSF), a monomeric glycoprotein functioning as a cytokine promoting both proliferation and activation of macrophages and neutrophils.
Abivax SA is a French clinical-stage, publicly traded biotechnology company harnessing the immune system to develop novel treatments against inflammatory diseases, viral diseases and cancer. The company is headquartered in Paris, France and closely cooperates with the CNRS Collaborative Laboratory in Montpellier, France.













