
The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

The western blot, or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination.
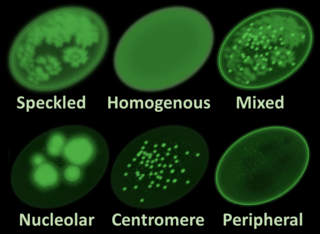
Antinuclear antibodies are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). In some cases, antibodies to human antigens are produced; these are known as autoantibodies.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.

Immunofluorescence(IF) is a light microscopy-based technique that allows detection and localization of a wide variety of target biomolecules within a cell or tissue at a quantitative level. The technique utilizes the binding specificity of antibodies and antigens. The specific region an antibody recognizes on an antigen is called an epitope. Several antibodies can recognize the same epitope but differ in their binding affinity. The antibody with the higher affinity for a specific epitope will surpass antibodies with a lower affinity for the same epitope.
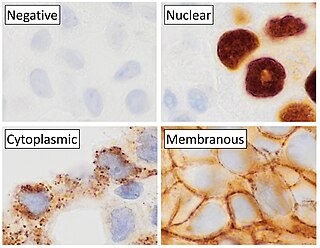
Immunohistochemistry is a form of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells and tissue, by exploiting the principle of antibodies binding specifically to antigens in biological tissues. Albert Hewett Coons, Ernest Berliner, Norman Jones and Hugh J Creech was the first to develop immunofluorescence in 1941. This led to the later development of immunohistochemistry.

Hybridoma technology is a method for producing large numbers of identical antibodies, also called monoclonal antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal myeloma cancer cells, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma.
The direct and indirect Coombs tests, also known as antiglobulin test (AGT), are blood tests used in immunohematology. The direct Coombs test detects antibodies that are stuck to the surface of the red blood cells. Since these antibodies sometimes destroy red blood cells they can cause anemia; this test can help clarify the condition. The indirect Coombs test detects antibodies that are floating freely in the blood. These antibodies could act against certain red blood cells; the test can be carried out to diagnose reactions to a blood transfusion.

Immunocytochemistry (ICC) is a common laboratory technique that is used to anatomically visualize the localization of a specific protein or antigen in cells by use of a specific primary antibody that binds to it. The primary antibody allows visualization of the protein under a fluorescence microscope when it is bound by a secondary antibody that has a conjugated fluorophore. ICC allows researchers to evaluate whether or not cells in a particular sample express the antigen in question. In cases where an immunopositive signal is found, ICC also allows researchers to determine which sub-cellular compartments are expressing the antigen.
In the fields of histology, pathology, and cell biology, fixation is the preservation of biological tissues from decay due to autolysis or putrefaction. It terminates any ongoing biochemical reactions and may also increase the treated tissues' mechanical strength or stability. Tissue fixation is a critical step in the preparation of histological sections, its broad objective being to preserve cells and tissue components and to do this in such a way as to allow for the preparation of thin, stained sections. This allows the investigation of the tissues' structure, which is determined by the shapes and sizes of such macromolecules as proteins and nucleic acids.

The enzyme horseradish peroxidase (HRP), found in the roots of horseradish, is used extensively in biochemistry applications. It is a metalloenzyme with many isoforms, of which the most studied type is C. It catalyzes the oxidation of various organic substrates by hydrogen peroxide.

Immunolabeling is a biochemical process that enables the detection and localization of an antigen to a particular site within a cell, tissue, or organ. Antigens are organic molecules, usually proteins, capable of binding to an antibody. These antigens can be visualized using a combination of antigen-specific antibody as well as a means of detection, called a tag, that is covalently linked to the antibody. If the immunolabeling process is meant to reveal information about a cell or its substructures, the process is called immunocytochemistry. Immunolabeling of larger structures is called immunohistochemistry.
In the diagnostic laboratory, virus infections can be confirmed by a myriad of methods. Diagnostic virology has changed rapidly due to the advent of molecular techniques and increased clinical sensitivity of serological assays.
Chromogenic in situ hybridization (CISH) is a cytogenetic technique that combines the chromogenic signal detection method of immunohistochemistry (IHC) techniques with in situ hybridization. It was developed around the year 2000 as an alternative to fluorescence in situ hybridization (FISH) for detection of HER-2/neu oncogene amplification. CISH is similar to FISH in that they are both in situ hybridization techniques used to detect the presence or absence of specific regions of DNA. However, CISH is much more practical in diagnostic laboratories because it uses bright-field microscopes rather than the more expensive and complicated fluorescence microscopes used in FISH.
Antigen retrieval is a non-enzymatic pretreatment for immunostaining to reduce or eliminate the chemical modifications caused by formalin fixation, through high temperature heating or strong alkaline solution (non-heating).
Virus quantification is counting or calculating the number of virus particles (virions) in a sample to determine the virus concentration. It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines, recombinant proteins using viral vectors, and viral antigens all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.
Primary and secondary antibodies are two groups of antibodies that are classified based on whether they bind to antigens or proteins directly or target another (primary) antibody that, in turn, is bound to an antigen or protein.

Immunogold labeling or immunogold staining (IGS) is a staining technique used in electron microscopy. This staining technique is an equivalent of the indirect immunofluorescence technique for visible light. Colloidal gold particles are most often attached to secondary antibodies which are in turn attached to primary antibodies designed to bind a specific antigen or other cell component. Gold is used for its high electron density which increases electron scatter to give high contrast 'dark spots'.

A neuronal lineage marker is an endogenous tag that is expressed in different cells along neurogenesis and differentiated cells such as neurons. It allows detection and identification of cells by using different techniques. A neuronal lineage marker can be either DNA, mRNA or RNA expressed in a cell of interest. It can also be a protein tag, as a partial protein, a protein or an epitope that discriminates between different cell types or different states of a common cell. An ideal marker is specific to a given cell type in normal conditions and/or during injury. Cell markers are very valuable tools for examining the function of cells in normal conditions as well as during disease. The discovery of various proteins specific to certain cells led to the production of cell-type-specific antibodies that have been used to identify cells.
The enzyme-linked immunosorbent spot (ELISpot) is a type of assay that focuses on quantitatively measuring the frequency of cytokine secretion for a single cell. The ELISpot Assay is also a form of immunostaining since it is classified as a technique that uses antibodies to detect a protein analyte, with the word analyte referring to any biological or chemical substance being identified or measured.