Related Research Articles

Cell death is the event of a biological cell ceasing to carry out its functions. This may be the result of the natural process of old cells dying and being replaced by new ones, as in programmed cell death, or may result from factors such as diseases, localized injury, or the death of the organism of which the cells are part. Apoptosis or Type I cell-death, and autophagy or Type II cell-death are both forms of programmed cell death, while necrosis is a non-physiological process that occurs as a result of infection or injury.

Indoleamine-pyrrole 2,3-dioxygenase (IDO or INDO EC 1.13.11.52) is a heme-containing enzyme physiologically expressed in a number of tissues and cells, such as the small intestine, lungs, female genital tract or placenta. In humans is encoded by the IDO1 gene. IDO is involved in tryptophan metabolism. It is one of three enzymes that catalyze the first and rate-limiting step in the kynurenine pathway, the O2-dependent oxidation of L-tryptophan to N-formylkynurenine, the others being indolamine-2,3-dioxygenase 2 (IDO2) and tryptophan 2,3-dioxygenase (TDO). IDO is an important part of the immune system and plays a part in natural defense against various pathogens. It is produced by the cells in response to inflammation and has an immunosuppressive function because of its ability to limit T-cell function and engage mechanisms of immune tolerance. Emerging evidence suggests that IDO becomes activated during tumor development, helping malignant cells escape eradication by the immune system. Expression of IDO has been described in a number of types of cancer, such as acute myeloid leukemia, ovarian cancer or colorectal cancer. IDO is part of the malignant transformation process and plays a key role in suppressing the anti-tumor immune response in the body, so inhibiting it could increase the effect of chemotherapy as well as other immunotherapeutic protocols.. Furthermore, there is data implicating a role for IDO1 in the modulation of vascular tone in conditions of inflammation via a novel pathway involving singlet oxygen.

Leukotriene C4 (LTC4) is a leukotriene. LTC4 has been extensively studied in the context of allergy and asthma. In cells of myeloid origin such as mast cells, its biosynthesis is orchestrated by translocation to the nuclear envelope along with co-localization of cytosolic phospholipase A2 (cPLA2), Arachidonate 5-lipoxygenase (5-LO), 5-lipoxygenase-activating protein (FLAP) and LTC4 synthase (LTC4S), which couples glutathione to an LTA4 intermediate. The MRP1 transporter then secretes cytosolic LTC4 and cell surface proteases further metabolize it by sequential cleavage of the γ-glutamyl and glycine residues off its glutathione segment, generating the more stable products LTD4 and LTE4. All three leukotrienes then bind at different affinities to two G-protein coupled receptors: CYSLTR1 and CYSLTR2, triggering pulmonary vasoconstriction and bronchoconstriction.

Macrophage migration inhibitory factor (MIF), also known as glycosylation-inhibiting factor (GIF), L-dopachrome isomerase, or phenylpyruvate tautomerase is a protein that in humans is encoded by the MIF gene. MIF is an important regulator of innate immunity. The MIF protein superfamily also includes a second member with functionally related properties, the D-dopachrome tautomerase (D-DT). CD74 is a surface receptor for MIF.
T helper 17 cells (Th17) are a subset of pro-inflammatory T helper cells defined by their production of interleukin 17 (IL-17). They are related to T regulatory cells and the signals that cause Th17s to differentiate actually inhibit Treg differentiation. However, Th17s are developmentally distinct from Th1 and Th2 lineages. Th17 cells play an important role in maintaining mucosal barriers and contributing to pathogen clearance at mucosal surfaces; such protective and non-pathogenic Th17 cells have been termed as Treg17 cells.

Bruce Alan Beutler is an American immunologist and geneticist. Together with Jules A. Hoffmann, he received one-half of the 2011 Nobel Prize in Physiology or Medicine, for "discoveries concerning the activation of innate immunity." Beutler discovered the long-elusive receptor for lipopolysaccharide. He did so by identifying spontaneous mutations in the gene coding for mouse Toll-like receptor 4 (Tlr4) in two unrelated strains of LPS-refractory mice and proving they were responsible for that phenotype. Subsequently, and chiefly through the work of Shizuo Akira, other TLRs were shown to detect signature molecules of most infectious microbes, in each case triggering an innate immune response.

Triggering receptor expressed on myeloid cells 1 (TREM1) an immunoglobulin (Ig) superfamily transmembrane protein that, in humans, is encoded by the TREM1 gene. TREM1 is constitutively expressed on the surface of peripheral blood monocytes and neutrophils, and upregulated by toll-like receptor (TLR) ligands; activation of TREM1 amplifies immune responses.

Thymic stromal lymphopoietin (TSLP) is a protein belonging to the cytokine family. It is known to play an important role in the maturation of T cell populations through activation of antigen-presenting cells.

Hepatitis A virus cellular receptor 2 (HAVCR2), also known as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), is a protein that in humans is encoded by the HAVCR2 (TIM-3)gene. HAVCR2 was first described in 2002 as a cell surface molecule expressed on IFNγ producing CD4+ Th1 and CD8+ Tc1 cells. Later, the expression was detected in Th17 cells, regulatory T-cells, and innate immune cells. HAVCR2 receptor is a regulator of the immune response.

Interleukin-17A is a protein that in humans is encoded by the IL17A gene. In rodents, IL-17A used to be referred to as CTLA8, after the similarity with a viral gene.
The inflammatory reflex is a neural circuit that regulates the immune response to injury and invasion. All reflexes have an afferent and efferent arc. The Inflammatory reflex has a sensory afferent arc, which is activated by cytokines, and a motor or efferent arc, which transmits action potentials in the vagus nerve to suppress cytokine production. Increased signaling in the efferent arc inhibits inflammation and prevents organ damage.
The nuocyte is a cell of the innate immune system that plays an important role in type 2 immune responses that are induced in response to helminth worm infection or in conditions such as asthma and atopic disease. Nuocytes are amongst the first cells activated in type 2 immune responses and are thought to play important roles in activating and recruiting other cells types through their production of type 2 cytokines interleukin 4, 5 and 13. Nuocytes have been observed to proliferate in the presence of interleukin 7 (IL-7) in vitro. Nuocytes contribute to the expulsion of helminth worms and to the pathology of colitis and allergic airways disease.
Natural killer T (NKT) cells are a heterogeneous group of T cells that share properties of both T cells and natural killer cells. Many of these cells recognize the non-polymorphic CD1d molecule, an antigen-presenting molecule that binds self and foreign lipids and glycolipids. They constitute only approximately 1% of all peripheral blood T cells. Natural killer T cells should neither be confused with natural killer cells nor killer T cells.

Akiko Iwasaki is a Sterling Professor of Immunobiology and Molecular, Cellular and Developmental Biology at Yale University. She is also a principal investigator at the Howard Hughes Medical Institute. Her research interests include innate immunity, autophagy, inflammasomes, sexually transmitted infections, herpes simplex virus, human papillomavirus, respiratory virus infections, influenza infection, T cell immunity, commensal bacteria, COVID-19 and Long COVID.

ILC2 cells, or type 2 innate lymphoid cells are a type of innate lymphoid cell. Not to be confused with the ILC. They are derived from common lymphoid progenitor and belong to the lymphoid lineage. These cells lack antigen specific B or T cell receptor because of the lack of recombination activating gene. ILC2s produce type 2 cytokines and are involved in responses to helminths, allergens, some viruses, such as influenza virus and cancer.
In cell biology, TH9 cells are a sub-population of CD4+T cells that produce interleukin-9 (IL-9). They play a role in defense against helminth infections, in allergic responses, in autoimmunity, and tumor suppression.
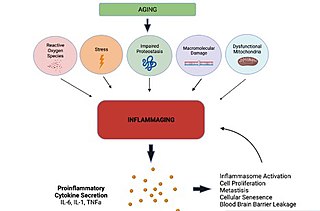
Inflammaging is a chronic, sterile low-grade inflammation that develops with advanced age, in the absence of overt infection, and may contribute to clinical manifestations of other age-related pathologies. Inflammaging is thought to be caused by a loss of control over systemic inflammation resulting in chronic, overstimulation of the innate immune system. Inflammaging is a significant risk factor in mortality and morbidity in aged individuals

Thirumala-Devi Kanneganti is an immunologist and is the Rose Marie Thomas Endowed Chair, Vice Chair of the Department of Immunology, and Member at St. Jude Children's Research Hospital. She is also Director of the Center of Excellence in Innate Immunity and Inflammation at St. Jude Children's Research Hospital. Her research interests include investigating fundamental mechanisms of innate immunity, including inflammasomes and inflammatory cell death, PANoptosis, in infectious and inflammatory disease and cancer.
Tania H. Watts is a Canadian Immunologist, Professor at the University of Toronto, past President of the Canadian Society for Immunology and from 2009-2019 held the Sanofi Pasteur Chair in Human Immunology at the University of Toronto. Tania Watts holds a Tier 1 Canada Research Chair in Anti-viral Immunity and was named a Distinguished Fellow of the American Association of Immunologists, class of 2022.
Type 2 inflammation is a pattern of immune response. Its physiological function is to defend the body against helminths, but a dysregulation of the type 2 inflammatory response has been implicated in the pathophysiology of several diseases.
References
- ↑ Murphy and Weaver, Kenneth and Casey (2016). Janeway's Immunobiology textbook. Taylor & Francis Ltd. ISBN 978-0-8153-4551-0.
- ↑ Nathan, Carl (2002). "Points of control in inflammation". Nature. 420 (6917): 846–852. Bibcode:2002Natur.420..846N. doi:10.1038/nature01320. PMID 12490957. S2CID 4426546.
- ↑ Kellum, John A.; Song, Mingchen; Li, Jinyou (2004-01-01). "Science review: Extracellular acidosis and the immune response: clinical and physiologic implications". Critical Care. 8 (5): 331–6. doi: 10.1186/cc2900 . ISSN 1364-8535. PMC 1065014 . PMID 15469594.
- ↑ Bogdan, Christian (2015-03-01). "Nitric oxide synthase in innate and adaptive immunity: an update". Trends in Immunology. 36 (3): 161–178. doi:10.1016/j.it.2015.01.003. ISSN 1471-4906. PMID 25687683.
- ↑ Nathan, Carl; Cunningham-Bussel, Amy (2013). "Beyond oxidative stress: an immunologist's guide to reactive oxygen species". Nature Reviews Immunology. 13 (5): 349–361. doi:10.1038/nri3423. PMC 4250048 . PMID 23618831.
- ↑ Machnik, Agnes; Neuhofer, Wolfgang; Jantsch, Jonathan; Dahlmann, Anke; Tammela, Tuomas; Machura, Katharina; Park, Joon-Keun; Beck, Franz-Xaver; Müller, Dominik N (2009). "Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism". Nature Medicine. 15 (5): 545–552. doi:10.1038/nm.1960. PMID 19412173. S2CID 10526891.
- ↑ Jantsch, Jonathan; Schatz, Valentin; Friedrich, Diana; Schröder, Agnes; Kopp, Christoph; Siegert, Isabel; Maronna, Andreas; Wendelborn, David; Linz, Peter (2015-03-03). "Cutaneous Na+ Storage Strengthens the Antimicrobial Barrier Function of the Skin and Boosts Macrophage-Driven Host Defense". Cell Metabolism. 21 (3): 493–501. doi:10.1016/j.cmet.2015.02.003. ISSN 1550-4131. PMC 4350016 . PMID 25738463.
- ↑ Kleinewietfeld, Markus; Manzel, Arndt; Titze, Jens; Kvakan, Heda; Yosef, Nir; Linker, Ralf A.; Muller, Dominik N.; Hafler, David A. (2013). "Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells". Nature. 496 (7446): 518–522. Bibcode:2013Natur.496..518K. doi:10.1038/nature11868. PMC 3746493 . PMID 23467095.
- ↑ Shapiro L and Dinarello CA (1995). "Osmotic regulation of cytokine synthesis in vitro". Proc Natl Acad Sci U S A. 92 (26): 12230–4. Bibcode:1995PNAS...9212230S. doi: 10.1073/pnas.92.26.12230 . PMC 40330 . PMID 8618875.
- ↑ Fay, Meredith E.; Myers, David R.; Kumar, Amit; Turbyfield, Cory T.; Byler, Rebecca; Crawford, Kaci; Mannino, Robert G.; Laohapant, Alvin; Tyburski, Erika A. (2016-02-23). "Cellular softening mediates leukocyte demargination and trafficking, thereby increasing clinical blood counts". Proceedings of the National Academy of Sciences. 113 (8): 1987–1992. Bibcode:2016PNAS..113.1987F. doi: 10.1073/pnas.1508920113 . ISSN 0027-8424. PMC 4776450 . PMID 26858400.
- ↑ Riesner, Katarina; Shi, Yu; Jacobi, Angela; Kräter, Martin; Kalupa, Martina; McGearey, Aleixandria; Mertlitz, Sarah; Cordes, Steffen; Schrezenmeier, Jens-Florian (2017-04-06). "Initiation of acute graft-versus-host disease by angiogenesis". Blood. 129 (14): 2021–2032. doi: 10.1182/blood-2016-08-736314 . ISSN 0006-4971. PMID 28096092.
- ↑ Muñoz, Luis E.; Bilyy, Rostyslav; Biermann, Mona H. C.; Kienhöfer, Deborah; Maueröder, Christian; Hahn, Jonas; Brauner, Jan M.; Weidner, Daniela; Chen, Jin (2016-10-04). "Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation". Proceedings of the National Academy of Sciences. 113 (40): E5856–E5865. Bibcode:2016PNAS..113E5856M. doi: 10.1073/pnas.1602230113 . ISSN 0027-8424. PMC 5056044 . PMID 27647892.
- ↑ McCollough, Cynthia H.; Leng, Shuai; Yu, Lifeng; Fletcher, Joel G. (2015-08-24). "Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications". Radiology. 276 (3): 637–653. doi:10.1148/radiol.2015142631. ISSN 0033-8419. PMC 4557396 . PMID 26302388.
- ↑ Wlodkowic, Donald; Darzynkiewicz, Zbigniew (2011-01-01). "Rise of the Micromachines: Microfluidics and the Future of Cytometry". In Zbigniew Darzynkiewicz, Elena Holden, Alberto Orfao, William Telford and Donald Wlodkowic (ed.). Recent Advances in Cytometry, Part A - Instrumentation, Methods. pp. 105–125. doi:10.1016/b978-0-12-374912-3.00005-5. ISBN 9780123749123. PMC 3241275 . PMID 21704837.
{{cite book}}:|journal=ignored (help)CS1 maint: multiple names: editors list (link)