The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

5-(2-Aminopropyl)indole is an indole and phenethylamine derivative with empathogenic effects. Its preparation was first reported by Albert Hofmann in 1962. It is a designer drug that has been openly sold as a recreational drug by online vendors since 2011.

JWH-098 is a synthetic cannabinoid receptor agonist from the naphthoylindole family. It is the indole 2-methyl derivative of a closely related compound JWH-081, but has markedly different affinity for the CB1 and CB2 receptors. While JWH-081 is around ten fold selective for CB1 over CB2, in JWH-098 this is reversed, and it is around four times weaker than JWH-081 at CB1 while being six times more potent at CB2, giving it a slight selectivity for CB2 overall. This makes JWH-098 a good example of how methylation of the indole 2-position in the naphthoylindole series tends to increase CB2 affinity, but often at the expense of CB1 binding.
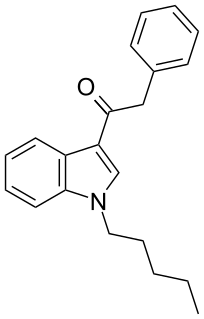
JWH-167 (1-pentyl-3-(phenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about 1.75 times selectivity for CB1 with a Ki of 90 nM ± 17 and 159 nM ± 14 at CB2. Similar to the related 2'-methoxy compound JWH-250, and the 2'-chloro compound JWH-203, JWH-167 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.
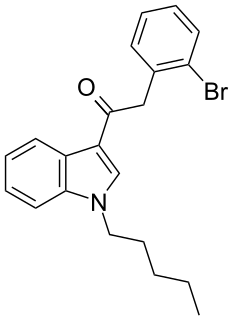
JWH-249 (1-pentyl-3-(2-bromophenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about 2.4 times selectivity for CB1 with a Ki of 8.4 ± 1.8 nM and 20 ± 2 nM at CB2. Similar to the related 2'-methoxy compound JWH-250, the 2'-chloro compound JWH-203, and the 2'-methyl compound JWH-251, JWH-249 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.

AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes.
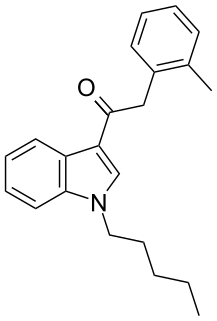
JWH-251 (1-pentyl-3-(2-methylphenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about five times selectivity for CB1 with a Ki of 29 nM and 146 nM at CB2. Similar to the related 2'-methoxy compound JWH-250, the 2'-chloro compound JWH-203, and the 2'-bromo compound JWH-249, JWH-251 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.

AM-1248 is a drug that acts as a moderately potent agonist for both the cannabinoid receptors CB1 and CB2, but with some dispute between sources over its exact potency and selectivity. Replacing the 3-(1-naphthoyl) group found in many indole derived cannabinoid ligands, with an adamantoyl group, generally confers significant CB2 selectivity, but reasonable CB1 affinity and selectivity is retained when an N-methylpiperidin-2-ylmethyl substitution is used at the indole 1-position. The related compound 1-pentyl-3-(1-adamantoyl)indole was identified as having been sold as a cannabinoid designer drug in Hungary in 2011, along with another synthetic cannabinoid AM-679.

Indole-5,6-quinone is an indolequinone, a chemical compound found in the oxidative browning reaction of fruits like bananas where it is mediated by the tyrosinase type polyphenol oxidase from tyrosine and catecholamines leading to the formation of catechol melanin. Like many quinones it can undergo redox reactions via the corresponding 5,6-dihydroxyindole.

JWH-302 (1-pentyl-3-(3-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family, which acts as a cannabinoid agonist with moderate affinity at both the CB1 and CB2 receptors. It is a positional isomer of the more common drug JWH-250, though it is slightly less potent with a Ki of 17 nM at CB1, compared to 11 nM for JWH-250. Because of their identical molecular weight and similar fragmentation patterns, JWH-302 and JWH-250 can be very difficult to distinguish by GC-MS testing.

1-(2-Dimethylaminoethyl)dihydropyrano(3,2-e)indole (4,5-DHP-DMT) is a tricyclic tryptamine derivative which acts as a potent and reasonably selective partial agonist for the serotonin receptor 5-HT2A, with a Ki of 17.0 nM, and moderate selectivity over related serotonin receptors. It has lower 5-HT2 affinity and efficacy than the related compound AL-37350A, but higher lipophilicity.
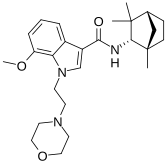
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

6-(2-Aminopropyl)indole is an indole derivative which was first identified being sold on the designer drug market by a laboratory in the Czech Republic in July 2016.
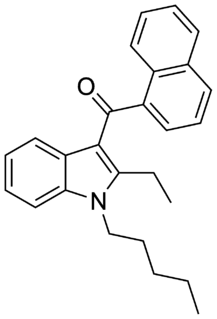
JWH-116 is a synthetic cannabinoid receptor ligand from the naphthoylindole family. It is the indole 2-ethyl derivative of related compound JWH-018. The binding affinity of JWH-116 for the CB1 receptor is reported as Ki = 52 ± 5 nM.
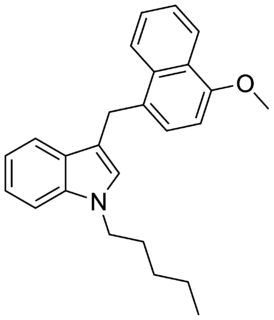
JWH-185 is a synthetic cannabinoid receptor ligand from the naphthoylindole family. It is the carbonyl-reduced derivative of related compound JWH-081. The binding affinity of JWH-185 for the CB1 receptor is reported as Ki = 17 ± 3 nM.

JWH-196 is a synthetic cannabinoid receptor ligand from the naphthylmethylindole family. It is the indole 2-methyl derivative of related compound JWH-175, and the carbonyl reduced analog of JWH-007. The binding affinity of JWH-196 for the CB1 receptor is reported as Ki = 151 ± 18 nM.
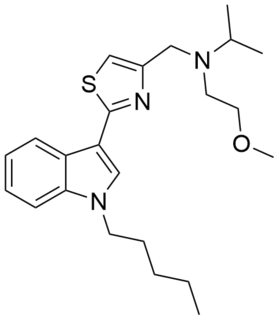
PTI-2 (SGT-49) is an indole-based synthetic cannabinoid. It is one of few synthetic cannabinoids containing a thiazole group and is closely related to PTI-1. These compounds may be viewed as simplified analogues of indole-3-heterocycle compounds originally developed by Organon and subsequently further researched by Merck.
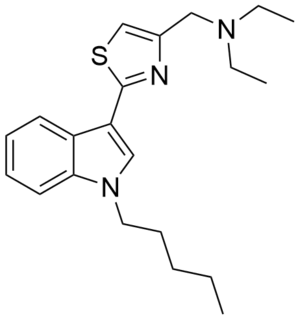
PTI-1 (SGT-48) is an indole-based synthetic cannabinoid. It is one of few synthetic cannabinoids containing a thiazole group and is closely related to PTI-2. These compounds may be viewed as simplified analogues of indole-3-heterocycle compounds originally developed by Organon and subsequently further researched by Merck.
The mitosenes are a class of organic chemicals based on a quinone-containing three-ring structure related to the two-ring core of the indolequinones. They are derived from the mitomycins by reduction and are the active alkylating agents responsible for the antitumor activity of the mitomycins.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.




















