
Current good manufacturing practices (cGMP) are those conforming to the guidelines recommended by relevant agencies. Those agencies control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use. The rules that govern each industry may differ significantly; however, the main purpose of GMP is always to prevent harm from occurring to the end user. Additional tenets include ensuring the end product is free from contamination, that it is consistent in its manufacture, that its manufacture has been well documented, that personnel are well trained, and that the product has been checked for quality more than just at the end phase. GMP is typically ensured through the effective use of a quality management system (QMS).
A subscription-based product of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), MedDRA or Medical Dictionary for Regulatory Activities is a clinically validated international medical terminology dictionary-thesaurus used by regulatory authorities and the biopharmaceutical industry during the regulatory process, from pre-marketing to post-marketing activities, and for safety information data entry, retrieval, evaluation, and presentation. Also, it is the adverse event classification dictionary.
Pharmacovigilance, also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: pharmakon and vigilare. As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
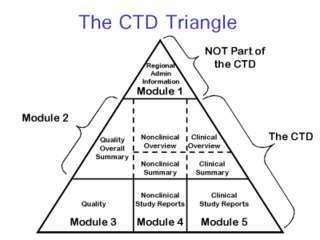
The Common Technical Document (CTD) is a set of specifications for an application dossier for the registration of medicine, designed for use across Europe, Japan, the United States, and beyond.

The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).
Regulatory affairs (RA), is a profession that deals with an organization’s adherence to regulatory compliance.
A serious adverse event (SAE) in human drug trials is defined as any untoward medical occurrence that at any dose
- Results in death
- Is life-threatening
- Requires inpatient hospitalization or causes prolongation of existing hospitalization
- Results in persistent or significant disability/incapacity
- May have caused a congenital anomaly/birth defect
- Requires intervention to prevent permanent impairment or damage
Good clinical practice (GCP) is an international quality standard, which governments can then transpose into regulations for clinical trials involving human subjects. GCP follows the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), and enforces tight guidelines on ethical aspects of clinical research.

The Council for International Organizations of Medical Sciences (CIOMS) is an international non-governmental organization of 40 international, national, and associate member groups representing the biomedical science community. It was jointly established by the World Health Organization (WHO) and United Nations Educational, Scientific and Cultural Organization (UNESCO) in 1949 as a successor to the International Medical Congress that organized 17 conferences from 1867 until the 1913 outbreak of World War One.
The electronic common technical document (eCTD) is an interface and international specification for the pharmaceutical industry to agency transfer of regulatory information. The specification is based on the Common Technical Document (CTD) format and was developed by the International Council for Harmonisation (ICH) Multidisciplinary Group 2 Expert Working Group.
In drug development and medical device development the Investigator's Brochure (IB) is a comprehensive document summarizing the body of information about an investigational product obtained during a drug trial. The IB is a document of critical importance throughout the drug development process and is updated with new information as it becomes available. The purpose of the IB is to compile data relevant to studies of the IP in human subjects gathered during preclinical and other clinical trials.
A clinical investigator involved in a clinical trial is responsible for ensuring that an investigation is conducted according to the signed investigator statement, the investigational plan, and applicable regulations; for protecting the rights, safety, and welfare of subjects under the investigator's care; and for the control of drugs under investigation. The Clinical Investigator must also meet requirements set forth by the FDA, EMA or other regulatory body. The qualifications must be outlined in a current resume and readily available for auditors.

The European Directorate for the Quality of Medicines & HealthCare (EDQM) is a Directorate of the Council of Europe that traces its origins and statutes to the Convention on the Elaboration of a European Pharmacopoeia.
Safety pharmacology is a branch of pharmacology specialising in detecting and investigating potential undesirable pharmacodynamic effects of new chemical entities (NCEs) on physiological functions in relation to exposure in the therapeutic range and above.
The following outline is provided as an overview of and topical guide to clinical research:
An estimand is a quantity that is to be estimated in a statistical analysis. The term is used to distinguish the target of inference from the method used to obtain an approximation of this target and the specific value obtained from a given method and dataset. For instance, a normally distributed random variable has two defining parameters, its mean and variance . A variance estimator:
In medicine, a clinical study report (CSR) on a clinical trial is a document, typically very long, providing much detail about the methods and results of a trial. A CSR is a scientific document addressing efficacy and safety, not a sales or marketing tool; its content is similar to that of a peer-reviewed academic paper. Results of trials are usually reported in a briefer academic journal paper, but methodological flaws are often glossed over in the briefer paper.
VigiBase is a World Health Organization's (WHO) global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO's international drug monitoring programme. It is the single largest drug safety data repository in the world. Since 1978, the Uppsala Monitoring Centre on behalf of WHO, have been maintaining VigiBase. Vigibase is used to obtain the information about a safety profile of a medicinal product. These data are used by pharmaceutical industries, academic institutions and regulatory authorities for statistical signal detection, updating periodic reports, ICSR comparisons with company databases and studying the reporting patterns. The data is collected from each of its 110 member states which currently comprises to over 10 million ICSRs. About a hundred thousand ICSRs are added each year.
Guidances for statistics in regulatory affairs refers to specific documents or guidelines that provide instructions, recommendations, and standards pertaining to the application of statistical methodologies and practices within the regulatory framework of industries such as pharmaceuticals and medical devices. These guidances serve as a reference for statisticians, researchers, and professionals involved in designing, conducting, analyzing, and reporting studies and trials in compliance with regulatory requirements. These documents embody the prevailing perspectives of regulatory agencies on specific subjects. It is worth noting that in the United States, the term "Guidances" is used, while in Europe, the term "Guidelines" is employed.