
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group. In organic chemistry, the acyl group is usually derived from a carboxylic acid, in which case it has the formula RCO−, where R represents an alkyl group that is linked to the carbon atom of the group by a single bond. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.

An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism.
The SN1 reaction is a substitution reaction in organic chemistry, the name of which refers to the Hughes-Ingold symbol of the mechanism. "SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular. Thus, the rate equation is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction occurs. In inorganic chemistry, the SN1 reaction is often known as the dissociative substitution. This dissociation pathway is well-described by the cis effect. A reaction mechanism was first proposed by Christopher Ingold et al. in 1940. This reaction does not depend much on the strength of the nucleophile, unlike the SN2 mechanism. This type of mechanism involves two steps. The first step is the ionization of alkyl halide in the presence of aqueous acetone or ethyl alcohol. This step provides a carbocation as an intermediate.
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.
A substitution reaction is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent.

Tandem mass spectrometry, also known as MS/MS or MS2, is a technique in instrumental analysis where two or more mass analyzers are coupled together using an additional reaction step to increase their abilities to analyse chemical samples. A common use of tandem MS is the analysis of biomolecules, such as proteins and peptides.
Infrared multiple photon dissociation (IRMPD) is a technique used in mass spectrometry to fragment molecules in the gas phase usually for structural analysis of the original (parent) molecule.
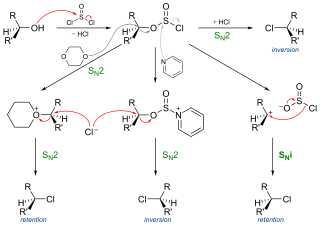
In chemistry, SNi refers to a specific but not often encountered reaction mechanism for nucleophilic aliphatic substitution. The name was introduced by Cowdrey et al. in 1937 to label nucleophilic reactions which occur with retention of configuration, but later was employed to describe various reactions that proceed with a similar mechanism.
Gas phase ion chemistry is a field of science encompassed within both chemistry and physics. It is the science that studies ions and molecules in the gas phase, most often enabled by some form of mass spectrometry. By far the most important applications for this science is in studying the thermodynamics and kinetics of reactions. For example, one application is in studying the thermodynamics of the solvation of ions. Ions with small solvation spheres of 1, 2, 3... solvent molecules can be studied in the gas phase and then extrapolated to bulk solution.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.
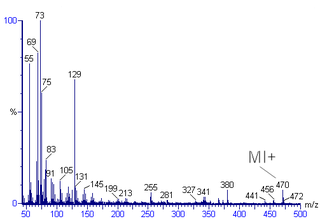
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It is commonly used for the identification of organic compounds from electron ionization mass spectrometry. Organic chemists obtain mass spectra of chemical compounds as part of structure elucidation and the analysis is part of many organic chemistry curricula.

Distonic ions are chemical species that contain two ionic charges on the same molecule, separated by two or more carbon or heteroatoms. A feature of distonic radical ions is that their charges and radical sites are in different locations, unlike regular radicals where the formal charge and unpaired electron are in the same location. These molecular species are created by ionization of either zwitterions or diradicals; ultimately, a neutral molecule loses an electron. Through experimental research distonic radicals have been found to be extremely stable gas phase ions and can be separated into different classes depending on the inherent features of the charged portion of the ion.

In mass spectrometry, fragmentation is the dissociation of energetically unstable molecular ions formed from passing the molecules in the ionization chamber of a mass spectrometer. The fragments of a molecule cause a unique pattern in the mass spectrum. These reactions are well documented over the decades and fragmentation pattern is useful to determine the molar weight and structural information of the unknown molecule. Fragmentation that occurs in tandem mass spectrometry experiments has been a recent focus of research, because this data helps facilitate the identification of molecules.
Photoelectron photoion coincidence spectroscopy (PEPICO) is a combination of photoionization mass spectrometry and photoelectron spectroscopy. It is largely based on the photoelectric effect. Free molecules from a gas-phase sample are ionized by incident vacuum ultraviolet (VUV) radiation. In the ensuing photoionization, a cation and a photoelectron are formed for each sample molecule. The mass of the photoion is determined by time-of-flight mass spectrometry, whereas, in current setups, photoelectrons are typically detected by velocity map imaging. Electron times-of-flight are three orders of magnitude smaller than those of ions, which allows electron detection to be used as a time stamp for the ionization event, starting the clock for the ion time-of-flight analysis. In contrast with pulsed experiments, such as REMPI, in which the light pulse must act as the time stamp, this allows to use continuous light sources, e.g. a discharge lamp or a synchrotron light source. No more than several ion–electron pairs are present simultaneously in the instrument, and the electron–ion pairs belonging to a single photoionization event can be identified and detected in delayed coincidence.
Hydrogen chalcogenides are binary compounds of hydrogen with chalcogen atoms. Water, the first chemical compound in this series, contains one oxygen atom and two hydrogen atoms, and is the most common compound on the Earth's surface.
In chemistry, the decay technique is a method to generate chemical species such as radicals, carbocations, and other potentially unstable covalent structures by radioactive decay of other compounds. For example, decay of a tritium-labeled molecule yields an ionized helium atom, which might then break off to leave a cationic molecular fragment.

Superelectrophilic anions are a class of molecular ions that exhibit highly electrophilic reaction behavior despite their overall negative charge. Thus, they are even able to bind the unreactive noble gases or molecular nitrogen at room temperature. The only representatives known so far are the fragment ions of the type [B12X11]– derived from the closo-dodecaborate dianions [B12X12]2–. X represents a substituent connected to a boron atom (cf. Fig. 1). For this reason, the following article deals exclusively with superelectrophilic anions of this type.