Agarose gel electrophoresis is a method of gel electrophoresis used in biochemistry, molecular biology, genetics, and clinical chemistry to separate a mixed population of macromolecules such as DNA or proteins in a matrix of agarose, one of the two main components of agar. The proteins may be separated by charge and/or size, and the DNA and RNA fragments by length. Biomolecules are separated by applying an electric field to move the charged molecules through an agarose matrix, and the biomolecules are separated by size in the agarose gel matrix.
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

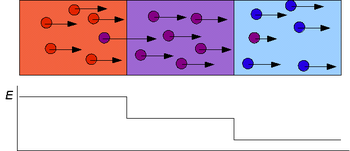
Gel electrophoresis is a method for separation and analysis of biomacromolecules and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation, and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc, which have been dissolved into liquid solutions.

Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium, namely agarose or polyacrylamide. Variants of gel electrophoresis include SDS-PAGE, free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each variant has many subtypes with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting or immunoblotting to give additional information about a specific protein.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
Micellar electrokinetic chromatography (MEKC) is a chromatography technique used in analytical chemistry. It is a modification of capillary electrophoresis (CE), extending its functionality to neutral analytes, where the samples are separated by differential partitioning between micelles and a surrounding aqueous buffer solution.
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions. Additionally, analytes may be concentrated or "focused" by means of gradients in conductivity and pH.
See Patented via Nth Cycle for metal electro-extraction process.
QPNC-PAGE, or Quantitative Preparative Native Continuous PolyAcrylamide Gel Electrophoresis, is a bioanalytical, one-dimensional, high-resolution and high-precision technique applied in biochemistry and bioinorganic chemistry to separate proteins quantitatively by isoelectric point and by continuous elution from a gel column. This standardized variant of native gel electrophoresis and subset of preparative polyacrylamide gel electrophoresis is used by biologists to isolate macromolecules in solution, for example, active or native metalloproteins in biological samples or properly and improperly folded metal cofactor-containing proteins or protein isoforms in complex protein mixtures.

Capillary electrophoresis–mass spectrometry (CE–MS) is an analytical chemistry technique formed by the combination of the liquid separation process of capillary electrophoresis with mass spectrometry. CE–MS combines advantages of both CE and MS to provide high separation efficiency and molecular mass information in a single analysis. It has high resolving power and sensitivity, requires minimal volume and can analyze at high speed. Ions are typically formed by electrospray ionization, but they can also be formed by matrix-assisted laser desorption/ionization or other ionization techniques. It has applications in basic research in proteomics and quantitative analysis of biomolecules as well as in clinical medicine. Since its introduction in 1987, new developments and applications have made CE-MS a powerful separation and identification technique. Use of CE–MS has increased for protein and peptides analysis and other biomolecules. However, the development of online CE–MS is not without challenges. Understanding of CE, the interface setup, ionization technique and mass detection system is important to tackle problems while coupling capillary electrophoresis to mass spectrometry.

Affinity electrophoresis is a general name for many analytical methods used in biochemistry and biotechnology. Both qualitative and quantitative information may be obtained through affinity electrophoresis. Cross electrophoresis, the first affinity electrophoresis method, was created by Nakamura et al. Enzyme-substrate complexes have been detected using cross electrophoresis. The methods include the so-called electrophoretic mobility shift assay, charge shift electrophoresis and affinity capillary electrophoresis. The methods are based on changes in the electrophoretic pattern of molecules through biospecific interaction or complex formation. The interaction or binding of a molecule, charged or uncharged, will normally change the electrophoretic properties of a molecule. Membrane proteins may be identified by a shift in mobility induced by a charged detergent. Nucleic acids or nucleic acid fragments may be characterized by their affinity to other molecules. The methods have been used for estimation of binding constants, as for instance in lectin affinity electrophoresis or characterization of molecules with specific features like glycan content or ligand binding. For enzymes and other ligand-binding proteins, one-dimensional electrophoresis similar to counter electrophoresis or to "rocket immunoelectrophoresis", affinity electrophoresis may be used as an alternative quantification of the protein. Some of the methods are similar to affinity chromatography by use of immobilized ligands.
The history of electrophoresis for molecular separation and chemical analysis began with the work of Arne Tiselius in 1931, while new separation processes and chemical analysis techniques based on electrophoresis continue to be developed in the 21st century. Tiselius, with support from the Rockefeller Foundation, developed the "Tiselius apparatus" for moving boundary electrophoresis, which was described in 1937 in the well-known paper "A New Apparatus for Electrophoretic Analysis of Colloidal Mixtures". The method spread slowly until the advent of effective zone electrophoresis methods in the 1940s and 1950s, which used filter paper or gels as supporting media. By the 1960s, increasingly sophisticated gel electrophoresis methods made it possible to separate biological molecules based on minute physical and chemical differences, helping to drive the rise of molecular biology. Gel electrophoresis and related techniques became the basis for a wide range of biochemical methods, such as protein fingerprinting, Southern blot, other blotting procedures, DNA sequencing, and many more.
Electrokinetics remediation, also termed electrokinetics, is a technique of using direct electric current to remove organic, inorganic and heavy metal particles from the soil by electric potential. The use of this technique provides an approach with minimum disturbance to the surface while treating subsurface contaminants.

In chemical analysis, capillary electrochromatography (CEC) is a chromatographic technique in which the mobile phase is driven through the chromatographic bed by electro-osmosis. Capillary electrochromatography is a combination of two analytical techniques, high-performance liquid chromatography and capillary electrophoresis. Capillary electrophoresis aims to separate analytes on the basis of their mass-to-charge ratio by passing a high voltage across ends of a capillary tube, which is filled with the analyte. High-performance liquid chromatography separates analytes by passing them, under high pressure, through a column filled with stationary phase. The interactions between the analytes and the stationary phase and mobile phase lead to the separation of the analytes. In capillary electrochromatography capillaries, packed with HPLC stationary phase, are subjected to a high voltage. Separation is achieved by electrophoretic migration of solutes and differential partitioning.
Free-flow electrophoresis (FFE), also known as carrier-free electrophoresis, is a matrix-free, high-voltage electrophoretic separation technique. FFE is an analogous technique to capillary electrophoresis, with a comparable resolution, that can be used for scientific questions, where semi-preparative and preparative amounts of samples are needed. It is used to quantitatively separate samples according to differences in charge or isoelectric point by forming a pH gradient. Because of the versatility of the technique, a wide range of protocols for the separation of samples like rare metal ions, protein isoforms, multiprotein complexes, peptides, organelles, cells, DNA origami, blood serum and nanoparticles exist. The advantage of FFE is the fast and gentle separation of samples dissolved in a liquid solvent without any need of a matrix, like polyacrylamide in gel electrophoresis. This ensures a very high recovery rate since analytes do not adhere to any carrier or matrix structure. Because of its continuous nature and high volume throughput, this technique allows a fast separation of preparative amounts of samples with a very high resolution. Furthermore, the separations can be conducted under native or denaturing conditions.
Electrostatic spray ionization (ESTASI) is an ambient ionization method for mass spectrometry (MS) analysis of samples located on a flat or porous surface, or inside a microchannel. It was developed in 2011 by Professor Hubert H. Girault’s group at the École Polytechnique Fédérale de Lausanne (EPFL) in Switzerland. In a typical ESTASI process, a droplet of a protic solvent containing analytes is deposited on a sample area of interest which itself is mounted to an insulating substrate. Under this substrate and right below the droplet, an electrode is placed and connected with a pulsed high voltage (HV) to electrostatically charge the droplet during pulsing. When the electrostatic pressure is larger than the surface tension, droplets and ions are sprayed. ESTASI is a contactless process based on capacitive coupling. One advantage of ESTASI is, that the electrode and sample droplet act contact-less avoiding thereby any oxidation or reduction of the sample compounds at the electrode surface, which often happens during standard electrospray ionization (ESI). ESTASI is a powerful new ambient ionization technique that has already found many applications in the detection of different analytes, such as organic molecules, peptides and proteins with molecule weight up to 70 kDa. Furthermore, it was used to couple MS with various separation techniques including capillary electrophoresis and gel isoelectric focusing, and it was successfully applied under atmospheric pressure to the direct analysis of samples with only few preparation steps.

Discontinuous electrophoresis is a type of polyacrylamide gel electrophoresis. It was developed by Ornstein and Davis. This method produces high resolution and good band definition. It is widely used technique for separating proteins according to size and charge.
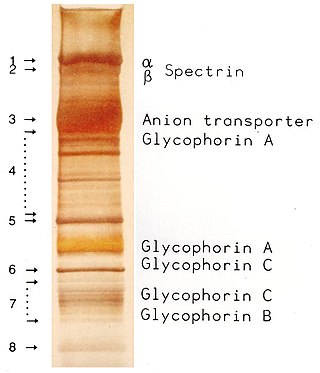
SDS-PAGE is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.