A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, cups, jars, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

Karl Waldemar Ziegler was a German chemist who won the Nobel Prize in Chemistry in 1963, with Giulio Natta, for work on polymers. The Nobel Committee recognized his "excellent work on organometallic compounds [which]...led to new polymerization reactions and ... paved the way for new and highly useful industrial processes". He is also known for his work involving free-radicals, many-membered rings, and organometallic compounds, as well as the development of Ziegler–Natta catalyst. One of many awards Ziegler received was the Werner von Siemens Ring in 1960 jointly with Otto Bayer and Walter Reppe, for expanding the scientific knowledge of and the technical development of new synthetic materials.
A post-metallocene catalyst is a kind of catalyst for the polymerization of olefins, i.e., the industrial production of some of the most common plastics. "Post-metallocene" refers to a class of homogeneous catalysts that are not metallocenes. This area has attracted much attention because the market for polyethylene, polypropylene, and related copolymers is large. There is a corresponding intense market for new processes as indicated by the fact that, in the US alone, 50,000 patents were issued between 1991-2007 on polyethylene and polypropylene.
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid and gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts.
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundations of many chemical industries.
John Paul Hogan was an American research chemist. Along with Robert Banks, he discovered methods of producing polypropylene and high-density polyethylene.
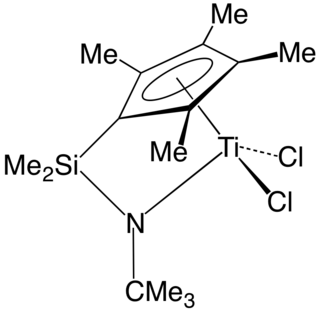
In organometallic chemistry, a "constrained geometry complex" (CGC) is a kind of catalyst used for the production of polyolefins such as polyethylene and polypropylene. The catalyst was one of the first major departures from metallocene-based catalysts and ushered in much innovation in the development of new plastics.

Robert Howard GrubbsForMemRS was an American chemist and the Victor and Elizabeth Atkins Professor of Chemistry at the California Institute of Technology in Pasadena, California. He was a co-recipient of the 2005 Nobel Prize in Chemistry for his work on olefin metathesis.
In polymer chemistry, ring-opening metathesis polymerization (ROMP) is a type of chain-growth polymerization involving olefin metathesis. The reaction is driven by relieving ring strain in cyclic olefins. A variety of heterogeneous and homogeneous catalysts have been developed for different polymers and mechanisms. Heterogeneous catalysts are typical in large-scale commercial processes, while homogeneous catalysts are used in finer laboratory chemical syntheses. Organometallic catalysts used in ROMP usually have transition metal centres, such as tungsten, rubidium, titanium, etc., with organic ligands.

John E. Bercaw is an American chemist and Centennial Professor of Chemistry, Emeritus at the California Institute of Technology.

Polymer Char is a company which designs and manufactures instrumentation for polymer analysis.
In polymer chemistry, chain walking (CW) or chain running or chain migration is a mechanism that operates during some alkene polymerization reactions. CW can be also considered as a specific case of intermolecular chain transfer. This reaction gives rise to branched and hyperbranched/dendritic hydrocarbon polymers. This process is also characterized by accurate control of polymer architecture and topology. The extent of CW, displayed in the number of branches formed and positions of branches on the polymers are controlled by the choice of a catalyst. The potential applications of polymers formed by this reaction are diverse, from drug delivery to phase transfer agents, nanomaterials, and catalysis.
Charles T. Kresge is a chemist and retired Chief Technology Officer (CTO) of Saudi Aramco. He was R&D Vice President at the Dow Chemical Company. His area of expertise is inorganic synthesis, and his primary field of research is in the area of crystalline aluminosilicate materials, particularly for the discovery of mesoporous molecular sieves.

The Charles Goodyear Medal is the highest honor conferred by the American Chemical Society, Rubber Division. Established in 1941, the award is named after Charles Goodyear, the discoverer of vulcanization, and consists of a gold medal, a framed certificate and prize money. The medal honors individuals for "outstanding invention, innovation, or development which has resulted in a significant change or contribution to the nature of the rubber industry". Awardees give a lecture at an ACS Rubber Division meeting, and publish a review of their work in the society's scientific journal Rubber Chemistry and Technology.
Samuel Emmett Horne Jr. was a research scientist at B. F. Goodrich noted for first synthesizing cis-1,4-polyisoprene, the main polymer contained in natural tree rubber, using Ziegler catalysis. Earlier attempts to produce synthetic rubber from isoprene had been unsuccessful, but in 1955, Horne prepared 98 percent cis-1,4-polyisoprene via the stereospecific polymerization of isoprene. The product of this reaction differs from natural rubber only slightly. It contains a small amount of cis-1,2-polyisoprene, but it is indistinguishable from natural rubber in its physical properties.

Herbert S. Eleuterio was an American industrial chemist noted for technical contributions to catalysis, polymerization, industrial research management, and science education. In particular, he discovered the olefin metathesis reaction and several novel fluoropolymers. Additionally, he explored techniques for research leadership, especially methods for fostering collaboration, globalization, and scientific creativity.
Functionalized polyolefins are olefin polymers with polar and nonpolar functionalities attached onto the polymer backbone. There has been an increased interest in functionalizing polyolefins due to their increased usage in everyday life. Polyolefins are virtually ubiquitous in everyday life, from consumer food packaging to biomedical applications; therefore, efforts must be made to study catalytic pathways towards the attachment of various functional groups onto polyolefins in order to affect the material's physical properties.
Herman Pines was a Russian Empire–born American chemist best known for his work with Vladimir Ipatieff on the catalytic conversion of high-octane aviation fuel. Because of his scientific contributions, new processes were developed for the isomerization of paraffins, the alkylation of aromatic compounds, and base-catalyzed organic reactions.









